-
Name
Isovaleraldehyde
- EINECS 209-691-5
- CAS No. 590-86-3
- Article Data433
- CAS DataBase
- Density 0.792 g/cm3
- Solubility 15 g/L (20 °C) in water
- Melting Point -60 °C
- Formula C5H10O
- Boiling Point 93.515 °C at 760 mmHg
- Molecular Weight 86.1338
- Flash Point -1.667 °C
- Transport Information UN 1989 3/PG 2
- Appearance Colorless liquid
- Safety 7-16-26-36-37/39
- Risk Codes 11-36-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F, Xi
Xi
- Synonyms Isovaleraldehyde;
- PSA 17.07000
- LogP 1.23140
Synthetic route

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(pyridine)(tricyclohexylphosphine)iridium(I) tetrakis[3,5-bis(trifluoromethyl)phenyl]borate; hydrogen In tetrahydrofuran at 23℃; for 0.5h; | 100% |
| With [Os(η(5)-C5,κ-N-Cp(N))(CH3CN)2]PF6 In tetrahydrofuran-d8 at 60℃; for 1.33333h; Inert atmosphere; | 93% |
| With {(η6-p-cymene)RuCl2}{[3-(3,5-diaza-1-azonia-7-phosphatricyclo[3.3.1.13,7]decan-1-yl)propyl]silanetriyltrioxy} supported on silica-coated ferrite nanoparticles In water at 150℃; under 5171.62 - 6205.94 Torr; for 14h; Inert atmosphere; Microwave irradiation; | 87% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride; 1,1,1,3',3',3'-hexafluoro-propanol at 20℃; for 20h; Catalytic behavior; Time; Inert atmosphere; | 99% |
| With boron trifluoride diethyl etherate In diethyl ether for 4h; Ambient temperature; | 91% |
| With dimethyl sulfoxide at 70℃; for 6h; | 90.78% |

| Conditions | Yield |
|---|---|
| With hydrogen; HSA-Rh(I) In water; pentane at 60℃; under 60800 Torr; for 24h; Hydroformylation; | 99% |
| With hydrogen; acetylacetonatodicarbonylrhodium(l); bis-2,6-(2,4-dimethylphenyl)-4-phenylphosphabenzene In toluene at 80℃; under 22501.8 Torr; for 4h; | 85% |
| With hydrogen at 100℃; under 18751.9 Torr; for 2h; Inert atmosphere; Autoclave; | 70% |

| Conditions | Yield |
|---|---|
| With chloral hydrate In hexane at 25℃; for 1.5h; Inert atmosphere; | 98% |
| With tellurium; sodium tetrahydroborate; water 1.) EtOH, 25 deg C, 30 min; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; silica gel for 0.05h; microwave irradiation; | 96% |
| With diethylammonium chlorochromate; acetic acid In water at 24.84℃; Kinetics; Darkness; | 96% |
| In neat (no solvent) at 20℃; for 0.0666667h; Microwave irradiation; | 94% |

| Conditions | Yield |
|---|---|
| 96% | |
| With hydrogen; tetra-(n-butyl)ammonium iodide In water at 110℃; under 15001.5 Torr; for 24h; chemoselective reaction; | 61% |
| With palladium; isopentanoyl chloride at 190 - 200℃; Hydrogenation; |

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With silica gel; copper(II) nitrate In tetrachloromethane for 0.25h; Ambient temperature; | 96% |

| Conditions | Yield |
|---|---|
| With N,N'-dichlorobis(2,4,6-trichlorodiphenyl)urea; water In acetonitrile at 20℃; for 0.416667h; | 96% |

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With 1-benzyl-4-aza-1-azoniabicyclo[2.2.2]octane tribromide In methanol; dichloromethane at 20℃; for 0.133333h; | 95% |
-
-
123-51-3
i-Amyl alcohol

-
A
-
13423-15-9
3-methyltetrahydrofuran

-
B
-
513-38-2
Isobutyl iodide

-
C
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In chlorobenzene for 2h; Irradiation; | A 94% B 5% C 1% |


| Conditions | Yield |
|---|---|
| With dimethyl sulfoxide for 0.0347222h; Kornblum oxidation; Microwave irradiation; | 93% |
| With air; water; vanadia at 370 - 400℃; Reagens 4: Bimsstein; |
-
-
69824-23-3
2-(2-methylpropyl)-1,3-dithiane

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With silica gel; copper(II) nitrate In tetrachloromethane for 0.416667h; Ambient temperature; | 93% |

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With 9-borabicyclo[3.3.1]nonane dimer In tetrahydrofuran for 1h; Ambient temperature; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-Methylbutenoic acid With triethylsilane; decacarbonyldirhenium(0) In diethyl ether at 20℃; for 9h; Inert atmosphere; Schlenk technique; UV-irradiation; Stage #2: With hydrogenchloride; water In diethyl ether at 20℃; for 3h; Inert atmosphere; Schlenk technique; | 91% |

| Conditions | Yield |
|---|---|
| With water; N-bromobis(p-toluenesulfonyl)amine for 0.0416667h; Microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With 9-borabicyclo[3.3.1]nonane dimer; lithium dihydrido borata-bicyclo[3.3.0]nonane In tetrahydrofuran for 1h; Ambient temperature; | 89% |
| With zinc at 250℃; | |
| With calcium carbonate at 450 - 500℃; |

| Conditions | Yield |
|---|---|
| With 9-borabicyclo[3.3.1]nonane dimer In tetrahydrofuran for 1h; Ambient temperature; | 88% |

| Conditions | Yield |
|---|---|
| In hexane for 0.166667h; Ambient temperature; | 77% |
-
-
18804-59-6
isovaleraldehyde phenylhydrazone

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With phosphoric acid for 0.00333333h; microwave irradiation; | 74% |

| Conditions | Yield |
|---|---|
| In water pH=7; Neutral conditions; Photolysis; Inert atmosphere; Cooling with ice; | 73% |
| With sodium hydroxide; N-bromoacetamide at 35℃; Rate constant; | 43% |
| With dipotassium peroxodisulfate; silver nitrate In water at 33℃; Rate constant; Thermodynamic data; Mechanism; ΔE(activ.); ΔH(activ.); ΔS(activ.); |

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With phosphoric acid for 0.00416667h; microwave irradiation; | 70% |

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With phosphoric acid for 0.00277778h; microwave irradiation; | 69% |

| Conditions | Yield |
|---|---|
| With lithium-tris(diethylamino)hydridoaluminate In tetrahydrofuran at -78℃; for 3h; Reduction; | 64% |
| With sodium tris(diethylamino)aluminum hydride In tetrahydrofuran; dodecane at -78℃; for 8h; | 62% |
-
-
1438-14-8
2-isopropyloxirane

-
-
1822-00-0
trimethylsilylmethyllithium

-
B
-
4798-45-2, 73262-52-9, 132486-36-3, 132486-37-4
4-methyl-pent-1-en-3-ol

-
C
-
104108-01-2
trans-(4-Methyl-2-pentenyl)trimethylsilane

-
D
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethylpiperidinyl-lithium In diethyl ether; hexane at 0 - 20℃; for 2h; Product distribution; Further Variations:; Solvents; | A 9% B 5% C 63% D 13% |


| Conditions | Yield |
|---|---|
| In toluene | A 60% B n/a |
-
-
50-00-0
formaldehyd

-
-
115-11-7
isobutene

-
A
-
16302-35-5
4-methyl-3,6-dihydro-2H-pyran

-
B
-
2270-61-3
4-methyl-3,4-dihydro-2H-pyran

-
C
-
2568-33-4
3-methyl-butane-1,3-diol

-
D
-
96-39-9
methylcyclopentadiene

-
E
-
219811-94-6
3,4,4-trimethylcyclohexene

-
F
-
590-86-3
isovaleraldehyde

-
G
-
78-79-5
isoprene

| Conditions | Yield |
|---|---|
| With niobium phosphate at 299.84℃; for 3.33333h; Temperature; | A n/a B n/a C n/a D n/a E n/a F n/a G 57% |


| Conditions | Yield |
|---|---|
| Stage #1: With dimethyl sulfoxide In tetrahydrofuran at -30℃; for 0.5h; Swern oxidation; Stage #2: 2-methyl-propan-1-ol With triethylamine In tetrahydrofuran at -30 - 20℃; Swern oxidation; | 40% |

| Conditions | Yield |
|---|---|
| With rhodium(III) iodide; hydrogen In tetrahydrofuran at 150℃; under 45004.5 Torr; for 3h; Autoclave; Inert atmosphere; | A 40% B 29% |
| With 2-iodo-propane; rhodium(III) iodide; hydrogen In tetrahydrofuran at 150℃; under 45004.5 Torr; for 3h; Reagent/catalyst; Autoclave; Inert atmosphere; | A 24% B 36% |

-
-
106-98-9
1-butylene

-
-
590-18-1
(Z)-2-Butene

-
-
624-64-6
trans-2-Butene

-
-
75-28-5
Isobutane

-
-
201230-82-2
carbon monoxide

-
-
106-97-8
n-butane

-
A
-
110-62-3
pentanal

-
B
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| Stage #1: carbon monoxide With hydrogen; acetylacetonatodicarbonylrhodium(l); C56H44N4O2P2 In toluene at 90℃; under 7500.75 Torr; for 1h; Stage #2: 1-butylene; (Z)-2-Butene; trans-2-Butene; Isobutane; n-butane In toluene at 90℃; under 12751.3 Torr; for 4h; Product distribution / selectivity; | A 34% B n/a |
| Stage #1: carbon monoxide With hydrogen; acetylacetonatodicarbonylrhodium(l); C54H52N4O2P2 In toluene at 90℃; under 7500.75 Torr; for 1h; Stage #2: 1-butylene; (Z)-2-Butene; trans-2-Butene; Isobutane; n-butane In toluene at 90℃; under 12751.3 Torr; for 4h; Product distribution / selectivity; | A 28% B n/a |
| Stage #1: carbon monoxide With hydrogen; acetylacetonatodicarbonylrhodium(l); [5-bis(3-methylindol-1-yl)phosphanyloxy-2,7-di-tert-butyl-9,9-dimethyl-xanthen-4-yl]oxy-bis(3-methylindol-1-yl)phosphane In toluene at 90℃; under 7500.75 Torr; for 1h; Stage #2: 1-butylene; (Z)-2-Butene; trans-2-Butene; Isobutane; n-butane In toluene at 90℃; under 12751.3 Torr; for 4h; Product distribution / selectivity; | A 37 %Chromat. B n/a |


| Conditions | Yield |
|---|---|
| With [Ru(η(5)-C5,κ-N-Cp(N))(CH3CN)2]PF6 In tetrahydrofuran-d8 at 60℃; for 5.33333h; Inert atmosphere; | A 31% B 16% |
-
-
590-86-3
isovaleraldehyde

-
-
108-59-8
malonic acid dimethyl ester

-
-
53618-21-6
dimethyl 2-(3-methylbutylidene)malonate

| Conditions | Yield |
|---|---|
| With chromatorex NH In toluene at 70℃; Reagent/catalyst; Temperature; Knoevenagel Condensation; Flow reactor; Molecular sieve; | 100% |
| With L-proline In dimethyl sulfoxide at 20℃; Reagent/catalyst; Knoevenagel Condensation; | 97% |
| With rac-Pro-OH In dimethyl sulfoxide at 20℃; for 16h; Knoevenagel condensation; | 92% |
-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
590-86-3
isovaleraldehyde

-
-
34993-63-0
ethyl (E)-5-methylhex-2-enoate

| Conditions | Yield |
|---|---|
| Stage #1: diethoxyphosphoryl-acetic acid ethyl ester With sodium hydride In 1,2-dimethoxyethane at 0℃; for 0.416667h; Stage #2: isovaleraldehyde In 1,2-dimethoxyethane for 16h; Horner-Emmons homologation; Heating; | 100% |
| With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 6h; Wadsworth-Honer-Emmons olefination; | 95% |
| With sodium hydride In tetrahydrofuran | 90% |
-
-
101629-94-1
N-(1-benzylpropyl)-2<(tributylstannyl)methyl>propenamide

-
-
590-86-3
isovaleraldehyde

-
-
101629-95-2
4-Hydroxy-6-methyl-2-methylene-heptanoic acid ((S)-1-benzyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane at -78 - 0℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| In toluene at 50℃; for 1h; | 100% |
| In ethanol for 3h; Heating; | 81% |
-
-
176848-87-6
5-Trimethylsilanyl-4-trimethylsilanylmethyl-pent-3-enylamine

-
-
590-86-3
isovaleraldehyde

-
-
176848-89-8
[3-Methyl-but-(E)-ylidene]-(5-trimethylsilanyl-4-trimethylsilanylmethyl-pent-3-enyl)-amine

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve In tetrahydrofuran Ambient temperature; | 100% |
-
-
59983-39-0
(S)-1-amino-2-(methoxymethyl)pyrrolidine

-
-
590-86-3
isovaleraldehyde

-
-
72203-95-3
((S)-2-Methoxymethyl-pyrrolidin-1-yl)-[3-methyl-but-(E)-ylidene]-amine

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |
-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 3,6-dibromo-8-methoxyimidazo[1,2-a]pyrazine In tetrahydrofuran; hexane at -75℃; for 0.25h; Metallation; Stage #2: isovaleraldehyde In tetrahydrofuran; hexane at -75℃; for 0.25h; Addition; | 100% |
-
-
7524-52-9, 26988-71-6, 5619-09-0, 14907-27-8, 41222-70-2, 67557-19-1, 109492-62-8
(S)-tryptophan methyl ester hydrochloride

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| In toluene for 15h; Pictet-Spengler reaction; Heating; | 100% |
-
-
14907-27-8, 5619-09-0, 7524-52-9, 26988-71-6, 41222-70-2, 67557-19-1, 109492-62-8
(R)-(+)-tryptophan methyl ester hydrochloride

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| In toluene for 15h; Pictet-Spengler reaction; Heating; | 100% |
-
-
541-47-9
3-Methylbutenoic acid

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| In water for 0.5h; Passerini reaction; Sonication; Cooling with ice; | 100% |
| In water at 25℃; Kinetics; Further Variations:; Solvents; Reagents; Passerini reaction; | 95% |
| In water at 20℃; Passerini reaction; | 86% |
| In dichloromethane at 25℃; for 18h; Kinetics; Product distribution; Further Variations:; Solvents; Passerini reaction; | 45% |


| Conditions | Yield |
|---|---|
| With iron(III) chloride | 100% |

-
-
590-86-3
isovaleraldehyde

-
-
913618-91-4
5-methyl-1-triisopropylsilanyl-hex-1-yn-3-ol

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at -78℃; | 100% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate In benzene at 0℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In water; isopropyl alcohol at 20℃; for 0.166667h; | 100% |
| With formic acid; Au/TiO2-R In water at 80℃; under 760.051 Torr; for 9h; Inert atmosphere; Green chemistry; chemoselective reaction; | 85% |
| Stage #1: nitrobenzene With borane-ammonia complex; Pd/Fe3O4/Pt(111) In methanol at 20℃; under 2068.65 Torr; Flow reactor; Stage #2: isovaleraldehyde With acetic acid In methanol Flow reactor; Stage #3: With borane-ammonia complex In methanol Catalytic behavior; Flow reactor; | 85% |
| Stage #1: nitrobenzene With hydrogen In methanol at 20℃; under 760.051 Torr; for 5.5h; Stage #2: isovaleraldehyde With hydrogen In methanol at 20℃; under 760.051 Torr; for 5h; | 75% |
| With 5% Au/Fe2O3; hydrogen In toluene at 120℃; under 15001.5 Torr; for 6h; Autoclave; | 58% |
-
-
2687-43-6
O-benzylhydoxylamine hydrochloride

-
-
590-86-3
isovaleraldehyde

-
-
72399-25-8
3-methylbutyraldehyde O-benzyl-oxime

| Conditions | Yield |
|---|---|
| With pyridine at 80℃; for 2h; Inert atmosphere; | 100% |
| With pyridine In ethanol at 20℃; for 4h; | 86% |
| In pyridine at 80℃; for 2h; | |
| With sodium acetate In methanol; water at 21 - 24℃; for 2h; |

| Conditions | Yield |
|---|---|
| addn. of tributyltin alkoxide to aldehyde; | 100% |
| addn. of tributyltin alkoxide to aldehyde; | 100% |

| Conditions | Yield |
|---|---|
| With : H-D-Pro-Pro-Glu supported on polyethyleneglycol-polystyrene (TentaGel resin) In chloroform; isopropyl alcohol at -15℃; for 72h; optical yield given as %ee; enantioselective reaction; | 100% |
| With 4-methyl-morpholine; C16H26N4O5 In neat (no solvent) at 20℃; for 72h; stereoselective reaction; | 96% |
| With 4-methyl-morpholine; H-D-Pro-L-Pro-L-Glu-NH2*TFA In chloroform; isopropyl alcohol at 20℃; for 48h; optical yield given as %ee; stereoselective reaction; | 93% |
-
-
35458-21-0
phlorisobutyrophenone

-
-
77744-52-6
5-hydroxy-2,2,6,6-tetramethyl-cyclohex-4-ene-1,3-dione

-
-
590-86-3
isovaleraldehyde

-
-
1083197-75-4
5-hydroxy-2,2,6,6-tetramethyl-4-(3-methyl-1-(2,4,6-trihydroxy-3-isobutyrylphenyl)butyl)cyclohex-4-ene-1,3-dione

| Conditions | Yield |
|---|---|
| Stage #1: 5-hydroxy-2,2,6,6-tetramethyl-cyclohex-4-ene-1,3-dione; isovaleraldehyde With piperidine In dichloromethane at 20℃; for 0.25h; Mannich reaction; Stage #2: With hydrogenchloride; ammonium chloride In water Stage #3: phlorisobutyrophenone With sodium hydride In tetrahydrofuran at 20℃; for 3h; Friedel Crafts alkylation; | 100% |

-
-
541-47-9
3-Methylbutenoic acid

-
-
39546-47-9
p-chlorobenzylisocyanide

-
-
590-86-3
isovaleraldehyde

-
-
1262749-10-9
C18H25ClN2O2

| Conditions | Yield |
|---|---|
| With ammonia In methanol; water at 130℃; for 1.5h; Ugi reaction; Microwave irradiation; microwave tube; | 100% |

-
-
1245643-16-6
4-(difluoromethyl)bicyclo[2.2.2]octan-1-amine

-
-
590-86-3
isovaleraldehyde

-
-
1257397-61-7
4-(difluoromethyl)-N-isopentylbicyclo[2.2.2]octan-1-amine

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; Inert atmosphere; | 100% |
-
-
917-92-0
2,2-dimethyl-3-butyne

-
-
590-86-3
isovaleraldehyde

-
-
1226968-85-9
(±)-2,7,7-trimethyloct-5-yn-4-ol

| Conditions | Yield |
|---|---|
| Stage #1: 3,3-Dimethylbut-1-yne With n-butyllithium In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: isovaleraldehyde In tetrahydrofuran at -78 - 20℃; for 1.5h; Inert atmosphere; | 100% |
| Stage #1: 3,3-Dimethylbut-1-yne With n-butyllithium In tetrahydrofuran at -78 - 0℃; for 0.583333h; Inert atmosphere; Stage #2: isovaleraldehyde In tetrahydrofuran at -78 - 0℃; Inert atmosphere; |
-
-
848821-58-9
(2S)-2-{diphenyl[(trimethylsilyl)oxy]methyl}pyrrolidine

-
-
102-96-5
(2-nitroethenyl)benzene

-
-
590-86-3
isovaleraldehyde

| Conditions | Yield |
|---|---|
| With acetic acid In toluene at 25℃; | 100% |

-
-
590-86-3
isovaleraldehyde

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
-
94845-30-4
2-(isopentyloxy)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With [HC(CMeN(2,6-(isopropyl)2C6H3)2)]Al(hydride)(triflate) In benzene-d6 at 20℃; for 1h; Inert atmosphere; Sealed tube; | 100% |
| With catalyst:C4H9Mg(2,6-iPr2C6H3NCCH3)CH In benzene-d6 pinacolborane reacted with aldehyde in C6D6 at 25°C catalized by nBuMg(2,6-iPrC6H3NCCH3)2CH 0.1-0.5 mol% for <0.5 h; | |
| With 2-H-1,3-di-tert-butyl-2,3-dihydro-1H-1,3,2-diazaphosphole In [D3]acetonitrile at 20℃; for 9h; Catalytic behavior; Inert atmosphere; Sealed tube; | 88 %Spectr. |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 24h; | 100% |
Isovaleraldehyde Consensus Reports
Isovaleraldehyde Specification
The Isovaleraldehyde, with the CAS registry number 590-86-3, is also known as Butanal, 3-methyl-. It belongs to the classification code of Skin / Eye Irritant. Its EINECS registry number is 209-691-5. This chemical's molecular formula is C5H10O and molecular weight is 86.13. What's more, both its IUPAC name and systematic name are the same which is called 3-Methylbutanal. It should be stored in a cool, dry and well-ventilated place.
Physical properties about Isovaleraldehyde are: (1)ACD/LogP: 1.267; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.27; (4)ACD/LogD (pH 7.4): 1.27; (5)ACD/BCF (pH 5.5): 5.41; (6)ACD/BCF (pH 7.4): 5.41; (7)ACD/KOC (pH 5.5): 116.49; (8)ACD/KOC (pH 7.4): 116.49; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.383; (14)Molar Refractivity: 25.359 cm3; (15)Molar Volume: 108.764 cm3; (16)Polarizability: 10.053×10-24cm3; (17)Surface Tension: 22.675 dyne/cm; (18)Density: 0.792 g/cm3; (19)Flash Point: -1.667 °C; (20)Enthalpy of Vaporization: 33.327 kJ/mol; (21)Boiling Point: 93.515 °C at 760 mmHg; (22)Vapour Pressure: 49.317 mmHg at 25 °C.
Preparation of Isovaleraldehyde: this chemical can be prepared by 3-methyl-butyric acid. This reaction needs reagent zinc at temperature of 250 °C.

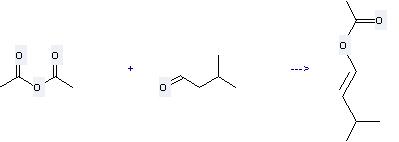
Uses of Isovaleraldehyde: (1) it is used as fruit type essence and intermediates; (2) it is used to produce other chemicals. For example, it can react with acetic acid anhydride to get acetic acid-(3-methyl-but-1-enyl ester). This reaction needs reagents K2CO3, KOAc at temperature of 130 °C. The reaction time is 2 hours. The yield is 60 %.
When you are dealing with this chemical, you should be very careful. This chemical may cause inflammation to the skin or other mucous membranes. It may catch fire in contact with air, only need brief contact with an ignition source and have a very low flash point or evolve highly flammable gases in contact with water. And it is irritating to eyes, respiratory system and skin. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. You must keep container tightly closed and keep away from sources of ignition. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=CCC(C)C
(2) InChI: InChI=1S/C5H10O/c1-5(2)3-4-6/h4-5H,3H2,1-2H3
(3) InChIKey: YGHRJJRRZDOVPD-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 2950mg/kg (2950mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 27(5), Pg. 60, 1983. | |
| guinea pig | LD50 | skin | > 8gm/kg (8000mg/kg) | Food and Chemical Toxicology. Vol. 26, Pg. 379, 1988. | |
| mouse | LC50 | inhalation | 50770mg/m3 (50770mg/m3) | BEHAVIORAL: EXCITEMENT LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 27(5), Pg. 60, 1983. |
| mouse | LD50 | oral | 4750mg/kg (4750mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 27(5), Pg. 60, 1983. | |
| mouse | LDLo | subcutaneous | 2gm/kg (2000mg/kg) | Annales Pharmaceutiques Francaises. Vol. 14, Pg. 710, 1956. | |
| rabbit | LD50 | skin | 3180uL/kg (3.18mL/kg) | LIVER: OTHER CHANGES SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | National Technical Information Service. Vol. OTS0535072, |
| rat | LC50 | inhalation | 42700mg/m3/4H (42700mg/m3) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS | National Technical Information Service. Vol. OTS0535072, |
| rat | LD50 | oral | 5600mg/kg (5600mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 31(12), Pg. 53, 1987. |
| rat | LDLo | intraperitoneal | 800mg/kg (800mg/kg) | Clinical Science. Vol. 61, Pg. 451, 1981. |
Related Products
- Isovaleraldehyde
- Isovaleraldehyde propyleneglycol acetal
- 59086-73-6
- 59086-90-7
- 5908-81-6
- 5908-87-2
- 59089-68-8
- 5908-99-6
- 590-90-9
- 590-92-1
- 5909-24-0
- 59092-91-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View