-
Name
Monoethyl fumarate
- EINECS 219-544-7
- CAS No. 2459-05-4
- Article Data44
- CAS DataBase
- Density 1.208 g/cm3
- Solubility Soluble in water.
- Melting Point 66-68 °C(lit.)
- Formula C6H8 O4
- Boiling Point 261.6 °C at 760 mmHg
- Molecular Weight 144.127
- Flash Point 109.6 °C
- Transport Information
- Appearance almost white to beige cryatalline power
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Butenedioicacid (2E)-, monoethyl ester (9CI);2-Butenedioic acid (E)-, monoethyl ester;Fumaric acid, ethyl ester (6CI,7CI);Fumaric acid, monoethyl ester (8CI);(E)-4-Ethoxy-4-oxo-2-butenoic acid;Ethyl hydrogen fumarate;Monoethylfumarate;Monoethyl trans-2-butenedioate;
- PSA 63.60000
- LogP 0.19030
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 8h; Ambient temperature; | 89% |
| With Candida antarctica lipase; water In 1,4-dioxane for 144h; | 78% |

| Conditions | Yield |
|---|---|
| With iron(III) perchlorate for 2h; Ambient temperature; | 87% |
-
-
124-38-9
carbon dioxide

-
-
1263187-14-9
3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-acrylic acid ethyl ester

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

| Conditions | Yield |
|---|---|
| With potassium methanolate; copper(l) chloride In N,N-dimethyl acetamide at 70℃; under 760.051 Torr; for 24h; Inert atmosphere; Schlenk technique; Sealed tube; | 76% |
-
-
1009307-13-4
ethyl (2E)-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)acrylate

-
-
124-38-9
carbon dioxide

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

| Conditions | Yield |
|---|---|
| With potassium methanolate; copper(l) chloride In N,N-dimethyl acetamide at 70℃; for 24h; Sealed tube; Schlenk technique; | 76% |
-
-
2459-05-4, 3249-53-4, 3990-03-2
malonic acid monoethyl ester

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride at 70℃; for 2h; | 58% |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
126580-11-8
ethyl 2-diazo-3-methyl-3-butenoate

-
A
-
2459-05-4, 3249-53-4, 3990-03-2
malonic acid monoethyl ester

-
B
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
C
-
1198173-63-5
diethyl 2-methylcyclobutene-1,3-dicarboxylate

| Conditions | Yield |
|---|---|
| With tetrakis(acetonitrile)copper(I)tetrafluoroborate In dichloromethane at 20℃; for 2h; Inert atmosphere; | A n/a B n/a C 33% |


| Conditions | Yield |
|---|---|
| With thionyl chloride | |
| With TEA | |
| Stage #1: maleic anhydride; ethanol at 60℃; for 3h; Stage #2: With aluminum (III) chloride at 20 - 90℃; for 2h; |
-
-
64-17-5
ethanol

-
-
617-48-1
malic acid

-
A
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
B
-
623-91-6
diethyl Fumarate

| Conditions | Yield |
|---|---|
| With hydrogenchloride |


| Conditions | Yield |
|---|---|
| at 120℃; | |
| With hydrogenchloride |
-
-
64-17-5
ethanol

-
-
110-17-8
(2E)-but-2-enedioic acid

-
A
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
B
-
623-91-6
diethyl Fumarate

| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| at 120℃; |


| Conditions | Yield |
|---|---|
| With potassium hydroxide; ethanol Schuetteln, Filtrieren, Verdunsten des Filtrats, Loesen des Rueckstands in Wasser und Schuetteln mit Aether aus; der waessr.Loesung mit Salzsaeure Uebersaettigen und mit Aether Ausschuetteln; |
-
-
82639-48-3
1,3,5,7,9,10-hexahydro-3,7,10-trimethylpyrimido<5,4-g>pteridine-2,4,6,8-tetrone

-
-
623-91-6
diethyl Fumarate

-
A
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
B
-
82639-46-1
3,7,10-trimethyl-(1H,3H,7H,10H)-pyrimido<5-4-g>pteridine-2,4,6,8-tetrone

-
C
-
110-17-8
(2E)-but-2-enedioic acid

-
D
-
123-25-1
succinic acid diethyl ester

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 30℃; for 12h; Rate constant; pH=7.00; | A 19 % Spectr. B n/a C 10 % Spectr. D 32 % Chromat. |

-
-
7446-08-4
selenium(IV) oxide

-
-
123-25-1
succinic acid diethyl ester

-
A
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
B
-
623-91-6
diethyl Fumarate

| Conditions | Yield |
|---|---|
| at 170℃; |

-
-
924-44-7
glyoxylic acid ethyl ester

-
-
3095-95-2
diethylphosphonoacetic acid

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

| Conditions | Yield |
|---|---|
| Stage #1: diethylphosphonoacetic acid With sodium hydride In 1,2-dimethoxyethane at 20℃; Stage #2: glyoxylic acid ethyl ester In 1,2-dimethoxyethane at 20℃; Further stages.; |

| Conditions | Yield |
|---|---|
| iodine In ethanol |
-
-
107679-25-4
but-2-enedioic acid tert-butyl ester ethyl ester

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

| Conditions | Yield |
|---|---|
| With water Alkaline conditions; |
-
-
2459-05-4, 3249-53-4, 3990-03-2
malonic acid monoethyl ester

-
-
17356-08-0
thiourea

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

| Conditions | Yield |
|---|---|
| In water at 80 - 85℃; for 4h; | 112 g |
-
-
121-75-5
[(dimethoxyphosphinothioyl)thio]-butanedioic acid, diethyl ester

-
A
-
1190-28-9
malathion alpha-dicarboxylic acid

-
B
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
C
-
3344-12-5
S-(1,2-dicarboethoxyethyl)O,S-dimethyl phosphorodithioate

-
D
-
1190-29-0
malathion monocarboxylic acid

-
F
-
141-05-9
Diethyl maleate

| Conditions | Yield |
|---|---|
| With carboxylesterase from Escherichia coli IES-02 In aq. phosphate buffer at 37℃; for 1h; pH=7.5; Catalytic behavior; Enzymatic reaction; |

-
-
17102-64-6
Sorbyl alcohol

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
160386-62-9
ethyl (2E,4E)-hexa-2,4-dien-1-yl fumarate

| Conditions | Yield |
|---|---|
| With dmap; 2`,3`-dideoxycytidine In dichloromethane Ambient temperature; | 100% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; for 3.5h; | 90% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
186417-99-2
octa-4t,6t-dien-3-ol

-
-
186418-00-8
(E)-But-2-enedioic acid ethyl ester (2E,4E)-1-ethyl-hexa-2,4-dienyl ester

| Conditions | Yield |
|---|---|
| With dmap; 2`,3`-dideoxycytidine In dichloromethane Ambient temperature; | 100% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
104-94-9
4-methoxy-aniline

-
-
37904-20-4
(E)–4-((4-methoxyphenyl)amino)-4-oxobut-2-enoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With oxalyl dichloride; N,N-dimethyl-formamide In benzene at 20℃; for 4h; Inert atmosphere; Stage #2: 4-methoxy-aniline With caesium carbonate In tetrahydrofuran at 20℃; Inert atmosphere; Stage #3: With potassium hydroxide; water In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; | 100% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
100-01-6
4-nitro-aniline

-
-
37904-21-5
fumaric acid mono-N-(4-nitrophenyl)amide

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With oxalyl dichloride; N,N-dimethyl-formamide In benzene at 20℃; for 4h; Inert atmosphere; Stage #2: 4-nitro-aniline With caesium carbonate In tetrahydrofuran at 20℃; Inert atmosphere; Stage #3: With potassium hydroxide; water In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; | 100% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
937172-42-4
(-)-(4E,6Z)-(1'R,2R,3S)-1,2,3-trihydroxy-1,3-O-ethylidene-4,6-octadiene

-
-
937172-48-0
(-)-(4E,6Z)-(1'R,2R,3S)-1,3-dihydroxy-1,3-O-ethylidene-4,6-octadien-2-yl ethyl fumarate

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 0.166667h; Inert atmosphere; Stage #2: With dmap In dichloromethane at 20℃; for 0.166667h; Inert atmosphere; Stage #3: (-)-(4E,6Z)-(1'R,2R,3S)-1,2,3-trihydroxy-1,3-O-ethylidene-4,6-octadiene In dichloromethane for 1h; Inert atmosphere; Reflux; | 100% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
7770-45-8
4-hydroxy-1-naphthaldehyde

-
-
696-60-6
4-aminomethylphenol

| Conditions | Yield |
|---|---|
| With lithium chloride; sodium hydroxide In water at 50℃; under 7500.75 Torr; for 1h; pH=7; Inert atmosphere; Microwave irradiation; | 100% |

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
7770-45-8
4-hydroxy-1-naphthaldehyde

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| With lithium chloride; sodium hydroxide In water at 50℃; under 7500.75 Torr; for 1h; pH=7; Inert atmosphere; Microwave irradiation; | 100% |

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
65473-13-4
N-methyl-1-naphthalenemethylamine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With 4-methyl-morpholine; isobutyl chloroformate In dichloromethane Stage #2: N-methyl-1-naphthalenemethylamine hydrochloride In dichloromethane Further stages.; | 99% |
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With 4-methyl-morpholine; isobutyl chloroformate In dichloromethane at -20℃; for 0.5h; Stage #2: N-methyl-1-naphthalenemethylamine hydrochloride In dichloromethane at 20℃; for 4h; Further stages.; | 99% |
-
-
110-89-4
piperidine

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
92912-62-4
trans-3-(1-piperidylcarbonyl)propenoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With 4-methyl-morpholine; isobutyl chloroformate In dichloromethane at -20℃; for 0.5h; Stage #2: piperidine In dichloromethane at 20℃; for 4h; Further stages.; | 99% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
100-61-8
N-methylaniline

-
-
1027101-46-7
(E)-4-(methylphenylamino)-4-oxo-2-butenoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With 4-methyl-morpholine; isobutyl chloroformate In dichloromethane at -20℃; for 0.5h; Stage #2: N-methylaniline In dichloromethane at 20℃; for 4h; Further stages.; | 99% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
103-67-3
benzyl-methyl-amine

-
-
945379-56-6
(E)-4-[methyl(phenylmethyl)amino]-4-oxo-2-butenoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 25℃; | 98% |
| 76% | |
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With 4-methyl-morpholine; isobutyl chloroformate In dichloromethane Stage #2: benzyl-methyl-amine In dichloromethane Further stages.; | 76% |
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With 4-methyl-morpholine; isobutyl chloroformate In dichloromethane at -20℃; for 0.5h; Stage #2: benzyl-methyl-amine In dichloromethane at 20℃; for 4h; Further stages.; | 76% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
26367-48-6
fumaric acid ethyl ester mono chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride at 20℃; for 24h; | 97% |
| With oxalyl dichloride In N,N-dimethyl-formamide | 93% |
| With dmap; oxalyl dichloride In dichloromethane at 20℃; for 1.5h; | 93% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
40893-97-8
(+/-)-2-(Bromomethyl)-1-butanol

-
-
155529-26-3
(+/-)-Ethyl (E)-3-((2-(bromomethyl)butoxy)carbonyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide | 97% |
| With dmap; dicyclohexyl-carbodiimide In dichloromethane for 2h; Ambient temperature; | 97% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
142039-01-8
Di(tert-butyl)-N-(3-aminopropyl)-N,N'-(ethan-1,2-diyl)bis

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 3h; | 97% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
610768-33-7
(2E)-3-(1-dimethylsulfamoyl-1H-imidazol-4-yl)prop-2-en-1-ol

-
-
610768-45-1
(2E)-2-butenedioic acid (2E)-3-(1-dimethylsulfamoyl-1H-imidazol-4-yl)-2-propenyl ester ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at -78 - 20℃; for 4h; | 97% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
88333-03-3, 10340-91-7
Benzyl isocyanide

-
-
630-19-3
pivalaldehyde

-
-
100-46-9
benzylamine

| Conditions | Yield |
|---|---|
| In dichloromethane at 200℃; under 13501.4 Torr; for 0.333333h; Ugi reaction; microwave irradiation; | 97% |

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
220378-49-4
C8H13N3O3S

-
-
610768-45-1
(2E)-2-butenedioic acid (2E)-3-(1-dimethylsulfamoyl-1H-imidazol-4-yl)-2-propenyl ester ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; Inert atmosphere; | 97% |
-
-
221044-05-9
N-(2-pyrimidyl)indole

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate; 2,2-dimethylpropanoic anhydride In toluene at 140℃; for 1h; Inert atmosphere; stereoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide at 55℃; for 16h; | 42.9 g |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
1310682-99-5
(-)-(E)-(1'R,2R,3S)-1,2,3-trihydroxy-1,3-O-ethylidene-5-methyl-4,6-heptadiene

-
-
1310683-04-5
(-)-(E)-(1'R,2R,3S)-1,3-dihydroxy-1,3-O-ethylidene-5-methyl-4,6-heptadien-2-yl ethyl fumarate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane for 1h; Inert atmosphere; Reflux; | 96% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -25 - 20℃; Solvent; Temperature; Time; Cooling with acetone-dry ice; | 96% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
88333-03-3, 10340-91-7
Benzyl isocyanide

-
-
66-99-9
β-naphthaldehyde

-
-
2393-23-9
4-methoxy-benzylamine

| Conditions | Yield |
|---|---|
| In dichloromethane at 200℃; under 13501.4 Torr; for 0.333333h; Ugi reaction; microwave irradiation; | 95% |

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
134855-87-1
α-methyl-p-hydroxybenzylamine

-
-
630-19-3
pivalaldehyde

| Conditions | Yield |
|---|---|
| In water at 200℃; under 13501.4 Torr; microwave irradiation; | 95% |

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
55096-88-3
N-benzyl-1-(4-fluorophenyl)methanamine

-
-
1027101-48-9
trans-3-(benzyl-4-fluorobenzyl)carbamoylpropenoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With 4-methyl-morpholine; isobutyl chloroformate In dichloromethane at -20℃; for 0.5h; Stage #2: N-benzyl-1-(4-fluorophenyl)methanamine In dichloromethane at 20℃; for 4h; Further stages.; | 95% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
111-92-2
dibutylamine

-
-
109447-28-1
trans-3-dibutylcarbamoylpropenoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With 4-methyl-morpholine; isobutyl chloroformate In dichloromethane at -20℃; for 0.5h; Stage #2: dibutylamine In dichloromethane at 20℃; for 4h; Further stages.; | 95% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
109-89-7
diethylamine

-
-
110793-11-8
trans-3-diethylcarbamoylpropenoic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (E)-4-ethoxy-4-oxobut-2-enoic acid With 4-methyl-morpholine; isobutyl chloroformate In dichloromethane at -20℃; for 0.5h; Stage #2: diethylamine In dichloromethane at 20℃; for 4h; Further stages.; | 95% |
-
-
110-91-8
morpholine

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
92912-63-5
(E)-4-(4-morpholinyl)-4-oxo-2-butenoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 25℃; | 95% |
-
-
1007217-08-4
(S)-3-amino-2-(tert-butyldiphenylsilanyloxy)propylcarbamic acid tert-butyl ester

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
1373889-07-6
C30H42N2O6Si

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 12h; Inert atmosphere; | 94% |
-
-
1189-71-5
isocyanate de chlorosulfonyle

-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
675876-18-3
(E)-4-ethoxy-4-oxobut-2-enoic anhydride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -25 - 10℃; for 6.5h; Solvent; Temperature; Time; Cooling with acetone-dry ice; | 94% |
-
-
2459-05-4
(E)-4-ethoxy-4-oxobut-2-enoic acid

-
-
3963-62-0
2,2-Diphenylethylamine

-
-
573986-11-5
fumaric acid mono-2,2-diphenylethylamide monoethyl ester

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane at 20℃; for 20h; | 93% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane | 85% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane for 24h; | 85% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 48h; Inert atmosphere; | 71% |
Monoethyl fumarate Chemical Properties
The molecular structure of Ethyl fumarate (CAS NO.2459-05-4):
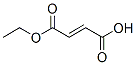
IUPAC Name: (E)-4-Ethoxy-4-oxobut-2-enoic acid
Molecular Formula: C6H8 O4
Molecular Weight: 144.1253 g/mol
Density: 1.208 g/cm3
Melting Point: 66-68 °C(lit.)
Boiling Point: 261.6 °C at 760 mmHg
Flash Point: 109.6 °C
Index of Refraction: 1.469
Molar Refractivity: 33.24 cm3
Molar Volume: 119.2 cm3
Polarizability: 13.17 ×10-24 cm3
Surface Tension: 42.4 dyne/cm
Enthalpy of Vaporization: 54.97 kJ/mol
Vapour Pressure: 0.0034 mmHg at 25 °C
XLogP3-AA: 0.2
H-Bond Donor: 1
H-Bond Acceptor: 4
Rotatable Bond Count: 4
Exact Mass: 144.042259
MonoIsotopic Mass: 144.042259
Topological Polar Surface Area: 63.6
Heavy Atom Count: 10
Canonical SMILES: CCOC(=O)C=CC(=O)O
Isomeric SMILES: CCOC(=O)/C=C/C(=O)O
InChI: InChI=1S/C6H8O4/c1-2-10-6(9)4-3-5(7)8/h3-4H,2H2,1H3,(H,7,8)/b4-3+
InChIKey: XLYMOEINVGRTEX-ONEGZZNKSA-N
EINECS: 219-544-7
Classification Code: dermatologic agents
Monoethyl fumarate Uses
Ethyl fumarate (CAS NO.2459-05-4) is used as preservative, polymer aggregation agent and food additive.
Monoethyl fumarate Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36: Wear suitable protective clothing.
WGK Germany: 1
HazardClass: IRRITANT
Monoethyl fumarate Specification
Ethyl fumarate (CAS NO.2459-05-4) is also named as Ethyl hydrogen fumarate ; Fumaric acid, monoethyl ester ; Monoethyl fumarate ; Monoethyl trans-2-butenedioate ; NSC 524101 ; 2-Butenedioic acid (E)-, monoethyl ester (9CI) ; Fumaric acid, ethyl ester . Ethyl fumarate (CAS NO.2459-05-4) is almost white to beige cryatalline power.
Related Products
- Monoethyl Adipate
- Monoethyl butylphosphonate anhydride with diethyl phosphate
- Monoethyl fumarate
- Monoethyl itaconate
- Monoethyl maleate
- Monoethyl phthalate
- Monoethyldichlorothiophosphate
- Monoethylphenyltriazene
- 2459-07-6
- 2459-09-8
- 2459-25-8
- 24593-59-7
- 24595-14-0
- 24596-18-7
- 24596-19-8
- 24598-62-7
- 24598-73-0
- 24599-21-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View