-
Name
Triacetin
- EINECS 203-051-9
- CAS No. 102-76-1
- Article Data140
- CAS DataBase
- Density 1.16 g/mL at 25 °C(lit.)
- Solubility 64.0 g/L (20 °C) in water
- Melting Point 3 °C(lit.)
- Formula C9H14O6
- Boiling Point 258 °C at 760 mmHg
- Molecular Weight 218.207
- Flash Point 93.9 °C
- Transport Information
- Appearance colourless liquid with a bitter taste
- Safety 23-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Triacetine;Triacetylglycerin;Triacetylglycerol;Ujostabil;Vanay;Triacetin(Food grade);Glycerol triacetate;1,2,3-Propanetriol,triacetate (9CI);Acetin, tri- (6CI,8CI);1,2,3-Triacetoxypropane;DRA 150;Edenor GTA;Enzactin;Estol 1581;Fungacetin;Glycerin triacetate;Glyceroltriacetate;Glyceryl triacetate;Glyped;Kesscoflex TRA;NSC 4796;Priacetin1580;Priacetin 1581;
- PSA 78.90000
- LogP 0.04430
Synthetic route

| Conditions | Yield |
|---|---|
| at 150℃; for 0.5h; | 99.5% |
| zirconium(IV) oxide at 200℃; for 2h; in autoclave; | 96% |
| With sulfonated charcoal In benzene for 7h; Heating; | 94% |

| Conditions | Yield |
|---|---|
| yttria-stabilized zirconia In acetonitrile for 8h; Heating; | 99% |
| With sodium hydroxide for 0.0166667h; microwave irradiation; | 99% |
| With SiO2-supported Co(II) Salen complex catalyst at 50℃; for 0.916667h; | 99% |

| Conditions | Yield |
|---|---|
| With C16H25N3O2S Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With aluminum oxide at 25℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With tetramethylammonium methyl carbonate for 15h; Molecular sieve; Reflux; Green chemistry; | 98% |
-
-
108-24-7
acetic anhydride

-
-
1708-40-3, 4141-19-9, 4141-20-2
cis-5-hydroxyl-2-phenyl-1,3-dioxane

-
-
102-76-1
triacetylglycerol

| Conditions | Yield |
|---|---|
| With erbium(III) triflate at 20℃; for 0.666667h; | 94% |

| Conditions | Yield |
|---|---|
| With zeolite HY at 20℃; for 11h; | 85% |

| Conditions | Yield |
|---|---|
| Multistep reaction.; | 60% |
-
-
96-11-7
1,2,3-tribromopropane

-
-
127-08-2
potassium acetate

-
A
-
63915-88-8
1-acetoxy-2-bromo-2-propene

-
B
-
102-76-1
triacetylglycerol

| Conditions | Yield |
|---|---|
| With acetic acid |

-
-
4704-87-4
2-bromo-1,3-propanediol

-
-
563-63-3
silver(I) acetate

-
-
64-19-7
acetic acid

-
-
102-76-1
triacetylglycerol

| Conditions | Yield |
|---|---|
| glycerol monobromohydrin of Fink; |


| Conditions | Yield |
|---|---|
| With potassium acetate |
-
-
108-05-4
vinyl acetate

-
-
105-70-4
2-hydroxypropane-1,3-diyl diacetate

-
-
121-44-8
triethylamine

-
-
102-76-1
triacetylglycerol

| Conditions | Yield |
|---|---|
| at 20℃; | |
| at 20℃; |


| Conditions | Yield |
|---|---|
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With sulfuric acid unter Abdestillieren des entstehenden Acetons; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid at 100 - 110℃; |

| Conditions | Yield |
|---|---|
| With pyridine 1.) hydrolysis; Multistep reaction; |
-
-
591-87-7
Allyl acetate

-
-
64-19-7
acetic acid

-
A
-
105-70-4
2-hydroxypropane-1,3-diyl diacetate

-
B
-
102-76-1
triacetylglycerol

| Conditions | Yield |
|---|---|
| With oxygen at 182℃; under 22501.8 Torr; nitrogen atmospherte; | |
| With oxygen under 22501.8 Torr; for 182h; Kinetics; nitrogen atmosphere, other temperature, other pressure; |


| Conditions | Yield |
|---|---|
| With tellurium oxide; lithium bromide at 120℃; for 20h; | 11.7 mmol |
-
-
201230-82-2
carbon monoxide

-
-
64-19-7
acetic acid

-
A
-
79-20-9
acetic acid methyl ester

-
B
-
111-55-7
ethylene glycol diacetate

-
C
-
102-76-1
triacetylglycerol

-
D
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| With dodecacarbonyl-triangulo-triruthenium; hydrogen at 260℃; under 258400 Torr; for 2h; Rate constant; Product distribution; different reaction conditions; | A 139 mmol B 1.58 mmol C n/a D n/a |

-
-
75-36-5
acetyl chloride

-
-
56-81-5
glycerol

-
A
-
105-70-4
2-hydroxypropane-1,3-diyl diacetate

-
B
-
93713-40-7
Glycerin-monoacetat

-
C
-
62108-08-1, 100-78-7
1,2,3-propanetriol 2-acetate

-
D
-
102-62-5
diacetin

-
E
-
102-76-1
triacetylglycerol

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; Product distribution; other acid chlorides; |

-
-
188637-14-1
1,2,3-triacetoxy-2-pivaloylpropane

-
-
102-76-1
triacetylglycerol

| Conditions | Yield |
|---|---|
| With 2-methylpropan-2-thiol In toluene at 25℃; deacylation; Photolysis; | 93 % Chromat. |
-
-
188637-14-1
1,2,3-triacetoxy-2-pivaloylpropane

-
A
-
102-76-1
triacetylglycerol

| Conditions | Yield |
|---|---|
| at 25℃; Disproportionation; deacylation; Photolysis; Title compound not separated from byproducts; | A 40 % Chromat. B n/a C n/a |


| Conditions | Yield |
|---|---|
| at 100 - 110℃; |



| Conditions | Yield |
|---|---|
| at 100℃; |


| Conditions | Yield |
|---|---|
| With C16H25N3O2S at 23℃; for 24h; | 99% |
| at 60℃; for 0.5h; | |
| With 4Zn(2+)*O(2-)*3C14H8O4(2-)*4.2C2H8N2 In toluene at 50℃; for 3h; Activation energy; Catalytic behavior; Reagent/catalyst; Temperature; |

| Conditions | Yield |
|---|---|
| With C16H25N3O2S In methanol at 23℃; for 24h; | 99% |
-
-
102-76-1
triacetylglycerol

-
-
98-85-1, 13323-81-4
1-Phenylethanol

-
A
-
1445-91-6
(S)-1-phenylethanol

-
B
-
16197-92-5
(R)-1-phenethyl acetate

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B at 40℃; for 6h; Kinetics; Temperature; Resolution of racemate; Enzymatic reaction; enantioselective reaction; | A n/a B 98% |


| Conditions | Yield |
|---|---|
| With scandium tris(trifluoromethanesulfonate) at 150℃; for 24h; Sealed tube; | 86% |

-
-
102-76-1
triacetylglycerol

-
-
80-62-6
methacrylic acid methyl ester

-
-
7401-88-9
glyceryl tri(methyl)acrylate

| Conditions | Yield |
|---|---|
| In cyclohexane | 82% |
| With hydrogenchloride; lithium bromide In cyclohexane | |
| With hydrogenchloride In cyclohexane |


| Conditions | Yield |
|---|---|
| sodium methylate In ethylene glycol at 120 - 125℃; for 3h; Conversion of starting material; | 79% |

| Conditions | Yield |
|---|---|
| With lipase B from Candida antarctica immobilized on octyl agarose In aq. phosphate buffer at 20℃; for 3h; pH=5.5; Enzymatic reaction; | 74% |

| Conditions | Yield |
|---|---|
| With hafnium(IV) trifluoromethanesulfonate at 120℃; for 14h; Sealed tube; | 42% |

| Conditions | Yield |
|---|---|
| With indium(III) triflate; iron(III) chloride; tris(triphenylphosphine)ruthenium(II) chloride; hydrogen; triphenylphosphine In acetic acid at 180℃; under 37503.8 Torr; for 12h; | 15% |

| Conditions | Yield |
|---|---|
| With methanol at 55℃; for 3h; Kinetics; Reagent/catalyst; Time; | 13.6% |
| With thorium dioxide at 460℃; |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
67-56-1
methanol

-
-
102-76-1
triacetylglycerol

-
A
-
79-20-9
acetic acid methyl ester

-
B
-
56-81-5
glycerol


| Conditions | Yield |
|---|---|
| With sodium methylate; xylene und Erwaermen des Reaktionsgemisches mit Glycerin; |
-
-
537-39-3
trielaidin

-
-
102-76-1
triacetylglycerol

-
-
98168-52-6
2-hydroxy-3-[(9E)-9-octadecenoyloxy]propyl (9E)-9-octadecenoate

| Conditions | Yield |
|---|---|
| With sodium methylate; xylene Erwaermen des Reaktionsgemisches mit Glycerin; |

| Conditions | Yield |
|---|---|
| katalytische Umsetzung; |


| Conditions | Yield |
|---|---|
| With sodium methylate; xylene anschliessendes Behandeln mit Glycerin bei 60grad; |
-
-
102-76-1
triacetylglycerol

-
-
555-44-2
glyceroltripalmitate

-
-
502-52-3
hexadecanoic acid, 2-hydroxy-1,3-propanediyl ester

| Conditions | Yield |
|---|---|
| With sodium methylate; xylene und Behandeln des Reaktionsgemisches mit Glycerin; |
-
-
102-76-1
triacetylglycerol

-
-
2438-40-6
β-1,2,3-tris(heptadecanoyl)glycerol

-
-
71431-35-1
2-hydroxy-1,3-propanediyl heptadecanoate

| Conditions | Yield |
|---|---|
| With sodium methylate; xylene Erwaermen des Reaktionsgemisches mit Glycerin; |

| Conditions | Yield |
|---|---|
| With sodium methylate; xylene und Erwaermen des Reaktionsgemisches mit Glycerin; |
-
-
102-76-1
triacetylglycerol

-
-
129-43-1
1-hydroxyanthraquinone

-
-
43099-11-2
6-hydroxy-7H-benzo[de]anthracen-7-one

| Conditions | Yield |
|---|---|
| With sulfuric acid; aniline sulfate at 145℃; |
-
-
102-76-1
triacetylglycerol

-
-
131-09-9
2-chloroanthracene-9,10-dione

-
-
873992-70-2
5-chloro-benz[de]anthracen-7-one

| Conditions | Yield |
|---|---|
| With sulfuric acid; aniline sulfate at 140℃; |
Triacetin Chemical Properties
Product Name: Triacetin (CAS NO.102-76-1)
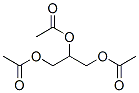
Molecular Formula: C9H14O6
Molecular Weight: 218.2g/mol
Mol File: 102-76-1.mol
Einecs: 3-051-9
Melting Point: 3 °C(lit.)
Boiling point: 258 °C at 760 mmHg
Flash Point: 93.9 °C
Density: 1.16 g/mL at 25 °C(lit.)
Refractive index: n25/D 1.429-1.431(lit.)
Water Solubility: 64.0 g/L (20 ºC)
Stability: Stable. Incompatible with strong oxidizing agents. Combustible.
Surface Tension: 36.4 dyne/cm
Enthalpy of Vaporization: 49.56 kJ/mol
Vapour Pressure: 0.0141 mmHg at 25°C
XLogP3-AA: 0.2
H-Bond Donor: 0
H-Bond Acceptor: 6
Structure Descriptors of Triacetin (CAS NO.102-76-1):
IUPAC Name: 1,3-diacetyloxypropan-2-yl acetate
Canonical SMILES: CC(=O)OCC(COC(=O)C)OC(=O)C
InChI: InChI=1S/C9H14O6/c1-6(10)13-4-9(15-8(3)12)5-14-7(2)11/h9H,4-5H2,1-3H3
InChIKey: URAYPUMNDPQOKB-UHFFFAOYSA-N
Product Categories: Functional Materials; Plasticizer; Polyalcohol Ethers, Esters (Plasticizer)
Triacetin Uses
Triacetin (CAS NO.102-76-1) is an artificial chemical compound, commonly used as a food additive, for instance as a solvent in flavourings, and for its humectant function, with E number E1518 and Australian approval code A1518. Triacetin is also a component of casting liquor with TG and as an excipient in pharmaceutical products where it is used as a humectant, a plasticizer, and as a solvent.
Triacetin can also be used as a fuel additive as an antiknock agent which can reduce engine knocking in gasoline, and to improve cold and viscosity properties of biodiesel.
Triacetin Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intravenous | 1500mg/kg (1500mg/kg) | Food and Cosmetics Toxicology. Vol. 16, Pg. 879, 1978. | |
| frog | LDLo | oral | 150mg/kg (150mg/kg) | Food and Cosmetics Toxicology. Vol. 16, Pg. 879, 1978. | |
| guinea pig | LDLo | intramuscular | 1740mg/kg (1740mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Journal of Pharmacology and Experimental Therapeutics. Vol. 76, Pg. 189, 1942. |
| mouse | LD50 | intraperitoneal | 1400mg/kg (1400mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: STIFFNESS | Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 22, Pg. 368, 1963. |
| mouse | LD50 | intravenous | 1600mg/kg (1600mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Acta Physiologica Scandinavica. Vol. 40, Pg. 338, 1957. |
| mouse | LD50 | oral | 1100mg/kg (1100mg/kg) | PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: STIFFNESS | Federation Proceedings, Federation of American Societies for Experimental Biology. Vol. 22, Pg. 368, 1963. |
| mouse | LD50 | subcutaneous | 2300mg/kg (2300mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Proceedings of the Society for Experimental Biology and Medicine. Vol. 46, Pg. 26, 1941. |
| rabbit | LD50 | intravenous | 750mg/kg (750mg/kg) | Food and Cosmetics Toxicology. Vol. 16, Pg. 879, 1978. | |
| rat | LD50 | intraperitoneal | 2100mg/kg (2100mg/kg) | Food and Cosmetics Toxicology. Vol. 16, Pg. 879, 1978. | |
| rat | LD50 | oral | 3gm/kg (3000mg/kg) | AMA Archives of Industrial Health. Vol. 21, Pg. 28, 1960. | |
| rat | LD50 | subcutaneous | 2800mg/kg (2800mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Proceedings of the Society for Experimental Biology and Medicine. Vol. 46, Pg. 26, 1941. |
Triacetin Safety Profile
Safety Information of Triacetin (CAS NO.102-76-1):
Safety Statements: 23-24/25
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
24: Avoid contact with skin
25: Avoid contact with eyes
Triacetin Specification
Triacetin ,its CAS NO. is 102-76-1,the synonyms is 1,2,3-Propanetriol triacetate ; 1,2,3-Propanetriyl triacetate ; 4-02-00-00253 (Beilstein Handbook Reference) ; AI3-00661 ; Acetin, tri- ; BRN 1792353 ; EINECS 203-051-9 ; Enzactin ; FEMA Number 2007 ; Fungacetin ; Glyceryl triacetate ; Glyped ; HSDB 585 ; Kesscoflex TRA ; Kodaflex triacetin ; NSC 4796 ; Triacetyl glycerine ; UNII-XHX3C3X673 ; Vanay .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View