-
Name
Triethylamine hydrochloride
- EINECS 209-067-2
- CAS No. 554-68-7
- Article Data337
- CAS DataBase
- Density 1.07 g/cm3
- Solubility 1440 g/L (20 °C) in water
- Melting Point 254-260 °C
- Formula C6H16ClN
- Boiling Point 90.5 °C at 760 mmHg
- Molecular Weight 137.653
- Flash Point 39 °C
- Transport Information
- Appearance White to off-white crystalline powder
- Safety 26-36-36/37/39-22
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  Xn
Xn
- Synonyms Triethylammonium chloride;Triethylamine monohydrochloride;N,N-diethylethanamine hydrochloride;Ethanamine, N,N-diethyl-, hydrochloride;Amine, triethyl, hydrochloride;Triethylamine hydrochloribe;
- PSA 3.24000
- LogP 2.15010
Synthetic route
-
-
109431-87-0
(R)-tert-butyl 3-hydroxypyrrolidine-1-carboxylate

-
-
124-63-0
methanesulfonyl chloride

-
-
121-44-8
triethylamine

-
A
-
554-68-7
triethylamine hydrochloride

-
B
-
127423-61-4
tert-butyl (3R)-3-methylsulfonyloxypyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| In toluene at 3 - 14℃; Product distribution / selectivity; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 1h; Cooling; | 99% |
| With hydrogenchloride In water at 0℃; for 1h; | 73% |
| With tetrachloromethane In benzene for 164h; Irradiation; | 0.5% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 80℃; for 8h; Heating / reflux; | A 99% B n/a |

-
-
2687-45-8
triethylamine N-oxide

-
-
97-00-7
1-chloro-2,4-dinitro-benzene

-
A
-
51-28-5
2,4-Dinitrophenol

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 24h; | A 98% B 98.5% |
| In N,N-dimethyl-formamide for 24h; Ambient temperature; | A 98% B 98.5% |


| Conditions | Yield |
|---|---|
| With triethylamine extn. with petroleum ether; | A n/a B 98% C n/a |

-
-
121-44-8
triethylamine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
927-74-2
1-butyn-4-ol

-
A
-
23418-85-1
3-butyn-1-yl p-toluenesulfonate

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 21h; | A 97% B n/a |


| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran | 97% |
-
-
41489-76-3
methyl-5-formyl-2-hydroxybenzoate

-
-
121-44-8
triethylamine

-
-
75-36-5
acetyl chloride

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: methyl-5-formyl-2-hydroxybenzoate; triethylamine In tetrahydrofuran for 0.0333333h; Stage #2: acetyl chloride at 25℃; for 2h; | A 97% B n/a |


| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran | 96% |
-
-
933-18-6
B,B',B''-trichloroborazine

-
-
121-44-8
triethylamine

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
A
-
113665-33-1
B,B',B''-tris{bis(trimethylsilyl)amino}borazine

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In benzene Addn. of mixt. of org. materials to a stirred soln. of B-compd. (C6H6) at room temp., stirring (room temp., 115 h).; Filtn. gives 96% of NEt3*HCl and 90% of borazine (obtained from filtrate). Purifn. by crystn. from hexane results in 71% pure material. Elem. anal.; | A 71% B 96% |

-
-
14794-31-1
ethyl 3-(chloroformyl)propionate

-
-
14691-88-4
4-amino-2,2,6,6-tetramethyl-1-piperidine-1-oxyl

-
A
-
554-68-7
triethylamine hydrochloride

-
B
-
82032-23-3
C15H27N2O4

| Conditions | Yield |
|---|---|
| With triethylamine In benzene 1.) 0 degC, 2.) R.T. 12 h; | A n/a B 95% |

-
-
794569-27-0
N,N-bis(trimethylsilyl)aminopropylmethyldichlorosilane

-
-
64-17-5
ethanol

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 20 - 30℃; for 3h; | A 95% B n/a |


-
-
22693-41-0
2,4,6-Triisopropylthiophenol

-
A
-
111975-83-8
{tungsten(C-t-Bu)(2,4,6-triisopropylbenzenethiolate)3Cl}{HNEt3}

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane byproducts: 1,2-dimethoxyethane; Slow addn. of solid W(C-t-Bu)Cl3(dme) to a stirred and cooled (-40°C) soln. of TIPTH and Et3N, soln. turns fisrt green, then red, warming (room temp.), stirring overnight.; Addn. of pentane, Et3NHCl pptd., filtn. through Celite, evapn. in vac. to dryness, elem. anal.; | A 95% B n/a |

-
-
527-69-5
2-furancarbonyl chloride

-
-
121-44-8
triethylamine

-
-
108-95-2
phenol

-
A
-
2948-14-3
furan-2-carboxylic acid phenyl ester

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 0.00416667h; Flow reactor; Green chemistry; | A 95% B n/a |

-
-
84115-18-4
ethyl α-chloro-α-nitro-β-hydroxypropionate

-
-
121-44-8
triethylamine

-
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In ethanol; water at 29℃; for 0.3h; Product distribution; | 94% |
-
-
67-56-1
methanol

-
-
794569-27-0
N,N-bis(trimethylsilyl)aminopropylmethyldichlorosilane

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 20 - 30℃; for 3h; | A 94% B n/a |

-
-
1541-10-2
mesitylthiol

-
A
-
111997-40-1
{tungsten(C-t-Bu)(2,4,6-trimethylbenzenethiolate)3Cl}{HNEt3}

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether byproducts: 1,2-dimethoxyethane; Slow addn. of solid W(C-t-Bu)Cl3(dme) to a stirred and cooled (-30°C) soln. of TMTH and Et3N, soln. first turns green, then yellow, warming (room temp.), stirring (90 min).; Addn. of pentane, filtn. through Celite, evapn. to dryness in vac., elem. anal.; | A 94% B n/a |

-
-
67-56-1
methanol

-
-
794569-27-0
N,N-bis(trimethylsilyl)aminopropylmethyldichlorosilane

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 20 - 30℃; for 3h; | A 92% B n/a |

-
-
61085-25-4, 76181-72-1
N-cyano-N'-methyl-N"-(2-mercaptoethyl)-guanidine

-
-
78-94-4
methyl vinyl ketone

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| With nitrogen; chlorine; triethylamine In chloroform | A 91% B n/a |
| With nitrogen; chlorine; triethylamine In chloroform | A 91% B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: diethylacetamide With bis(cyclopentadienyl)dihydrozirconium; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane at 20℃; under 760.051 Torr; for 12h; Inert atmosphere; Stage #2: With hydrogenchloride In diethyl ether Inert atmosphere; | 91% |
| Stage #1: diethylacetamide With [κ2-{Ph2P(Se)NC9H6N}Al(Me)2]; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane at 20℃; for 12h; Schlenk technique; Glovebox; Stage #2: With hydrogenchloride In water chemoselective reaction; | 90% |

-
-
13033-84-6
D-phenylalanine methyl ester hydrochloride

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: trans-4-isopropylcyclohexanecarboxylic acid; triethylamine With pivaloyl chloride In acetone at 5 - 20℃; for 3.5h; Stage #2: D-phenylalanine methyl ester hydrochloride In acetone at 20℃; Product distribution / selectivity; | A 90.07% B n/a |
| Stage #1: trans-4-isopropylcyclohexanecarboxylic acid; triethylamine With chloroformic acid ethyl ester In acetone at 0 - 20℃; for 3.5h; Heating / reflux; Stage #2: D-phenylalanine methyl ester hydrochloride In acetone at 25℃; Product distribution / selectivity; | A 78.42% B n/a |

-
-
622-40-2
2-(morpholin-4-yl)ethanol

-
-
2942-59-8
2-chloronicotinic acid

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride; triethylamine In toluene | A 90% B n/a |

-
-
98-88-4
benzoyl chloride

-
-
121-44-8
triethylamine

-
-
108-95-2
phenol

-
A
-
93-99-2
benzoic acid phenyl ester

-
B
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 0.00694444h; Flow reactor; Green chemistry; | A 90% B n/a |

-
-
50893-53-3
carbonochloridic acid 1-chloro-ethyl ester

-
-
121-44-8
triethylamine

-
-
75-08-1
ethanethiol

-
A
-
554-68-7
triethylamine hydrochloride

-
B
-
101506-43-8
O-(1-chloroethyl) S-ethyl carbonothioate

| Conditions | Yield |
|---|---|
| In diethyl ether at 0 - 20℃; for 5h; | A n/a B 89% |


-
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In methanol for 3h; | 100% |

-
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In acetonitrile (Ar); MeCN added to B compd. and N(C2H5)3HCl, stirred for 15 min; Et2O added carefully, crystd. for 5 d, dried (vac., overnight), H2O added, stirred for 15 min, sepd., washed (H2O), dried (vac.); | 100% |

| Conditions | Yield |
|---|---|
| at 60℃; for 4h; Inert atmosphere; Sealed tube; | 100% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-3-methylimidazolium bromide at 169.84℃; for 8h; | 99% |
-
-
554-68-7
triethylamine hydrochloride

-
-
616-38-6
carbonic acid dimethyl ester

-
-
10052-47-8
triethylmethylammonium chloride

| Conditions | Yield |
|---|---|
| With 1-ethyl-3-methylimidazolium bromide at 169.84℃; for 8h; | 99% |
| With 1-ethyl-3-methylimidazolium bromide at 169.84℃; for 8h; |
-
-
137003-15-7
(Bu4N)2[Fe4S4(1,3,5-tris-((4,6-dimethyl-3-mercaptophenyl)thio)-2,4,6-tris-(p-tolylthio)benzenate(3-))(SC2H5)]

-
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In acetonitrile byproducts: Et3N, EtSH; N2-atmosphere; stirring equimolar amts. for 12 h (periodic removal of Et3N and EtSH by evacuation); filtration, solvent removal (vac.); | 99% |

-
-
554-68-7
triethylamine hydrochloride

| Conditions | Yield |
|---|---|
| In acetonitrile byproducts: Et3N, EtSH; N2-atmosphere; stirring equimolar amts. for 12 h (periodic removal of Et3N and EtSH by evacuation); filtration, solvent removal (vac.); | 99% |
-
-
1266146-97-7
[(1,3-C6H3(CH2P(CF3)2)2)Ir(H)Cl(η2-C2H4)]

-
-
554-68-7
triethylamine hydrochloride

-
-
1268160-20-8
[(1,3-C6H3(CH2P(CF3)2)2)Ir(H)Cl2][HNEt3]

| Conditions | Yield |
|---|---|
| In benzene (N2); glovebox technique; mixt. of Ir complex (1 equiv.) and Et3NHCl (1 equiv.) in C6H6 was agitated at 20°C for 20 min; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine; aniline In water; benzene | 98% |
-
-
554-68-7
triethylamine hydrochloride

-
-
79-04-9
chloroacetyl chloride

-
-
173026-14-7
3,3-dimethyl-7-nitrobenzisoselenazoline

-
-
204272-78-6
N-(chloroacetyl)-3,3-dimethyl-7-nitrobenzisoselenazoline

| Conditions | Yield |
|---|---|
| With sodium chloride In tetrahydrofuran; water; ethyl acetate | 97% |

Triethylamine hydrochloride Consensus Reports
Triethylamine hydrochloride Specification
The Triethylamine hydrochloride with CAS registry number of 554-68-7 is also known as Ethanamine, N,N-diethyl-, hydrochloride. The IUPAC name is N,N-Diethylethanamine hydrochloride. It belongs to product categories of HPLC; HPLC buffer; HPLC buffers; HPLC Buffers-solutionchromatography/ce Reagents; Solution. Its EINECS registry number is 209-067-2. In addition, the formula is C6H16ClN and the molecular weight is 137.65. This chemical is a white to off-white crystalline powder and should be stored sealed containers.
Physical properties about Triethylamine hydrochloride are: (1)ACD/LogP: 1.66; (2)ACD/LogD (pH 5.5): -1.44; (3)ACD/LogD (pH 7.4): -1.2; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 3; (10)Flash Point: 39 °C; (11)Enthalpy of Vaporization: 31.01 kJ/mol; (12)Boiling Point: 90.5 °C at 760 mmHg; (13)Vapour Pressure: 56.1 mmHg at 25 °C.
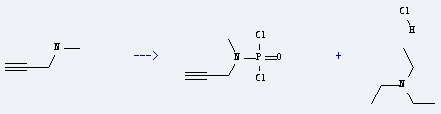
Preparation of Triethylamine hydrochloride: it is prepared by reaction of methyl-prop-2-ynyl-amine. The reaction needs reagents POCl3, (C2H5)3N at the temperature of 1-20 °C. The yield is about 59.8%.
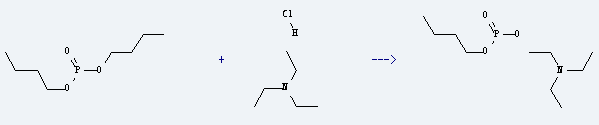
Uses of Triethylamine hydrochloride: it is used as raw material for quaternary ammonium salt, medicine, pesticides, dyes and other organic synthesis. It is used to produce triethylammonium salt of monobutyl phosphonate by reaction with phosphonic acid dibutyl ester. The reaction occurs at the temperature of 145 °C and the yield is about 85%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. Besides, it is harmful by inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing, gloves and eye/face protection. Do not breathe dust. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CCN(CC)CC.Cl
2. InChI: InChI=1S/C6H15N.ClH/c1-4-7(5-2)6-3;/h4-6H2,1-3H3;1H
3. InChIKey: ILWRPSCZWQJDMK-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | subcutaneous | 600mg/kg (600mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 37, Pg. 309, 1929. |
Related Products
- Triethylamine
- Triethylamine hydrobromide
- Triethylamine hydrochloride
- Triethylamine trihydrofluoride
- Triethylamine, 2,2,2-((phenylsilylidyne)trioxy)tris-
- Triethylamine,2-(p-mentha-6,8-dien-2-yloxy)- (8CI)
- Triethylamine,2,2'''-[(methylvinylene)bis(p-phenyleneoxy)]bis-, dihydrochloride (8CI)
- 55469-65-3
- 554-70-1
- 55473-29-5
- 554-73-4
- 55476-36-3
- 55477-35-5
- 55478-23-4
- 55478-55-2
- 55480-45-0
- 55-48-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View