-
Name
Ursodeoxycholic acid
- EINECS 204-879-3
- CAS No. 128-13-2
- Article Data64
- CAS DataBase
- Density 1.128 g/cm3
- Solubility practically insoluble in water
- Melting Point 203-204 °C(lit.)
- Formula C24H40O4
- Boiling Point 547.1 °C at 760 mmHg
- Molecular Weight 392.579
- Flash Point 298.8 °C
- Transport Information
- Appearance white crystalline powder
- Safety 24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms (4R)-4-[(3R,5S,7S,8R,9S,10R,13R,14S,17R)-3,7-dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoate;Cholan-24-oic acid, 3,7-dihydroxy-, (3-.alpha.,5-.beta., 7-.beta.)-;Cholan-24-oic acid,3,7-dihydroxy-,(3R,5a,- 7a)-;UDCA;5.beta.-Chol-24-oic acid-3.alpha., 7.beta.-diol;Arsacol;Ursochol;Antigall;Urso (TN);Ursodiol;Antigall (TN);Actigall;Urso;Litursol;Deursil;Desocol;Ursodeoxycholic acid (JP14);Delursan;Ursodiol (USP);deoxy-;Urso Deoxy Cholic Acid;7beta-Hydroxylithocholic acid;Ursodeoxycholoc acid;
- PSA 77.76000
- LogP 4.47790
Synthetic route
-
-
10538-55-3
3α,7β-dihydroxy-5β-cholan-24-oic acid methyl ester

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With methanol; sodium methylate at 25℃; | 99% |
| With water; sodium hydroxide at 97℃; for 2h; Large scale; | 49.1 kg |
| With water; sodium hydroxide at 120℃; for 12h; | 20.7 g |
| With water; sodium hydroxide at 120℃; for 12h; Time; | 29.4 g |

| Conditions | Yield |
|---|---|
| With glucose dehydrogenase; D-Glucose; NADPH; 7β-hydroxysteroid dehydrogenase In aq. phosphate buffer at 30℃; for 1h; pH=8; Concentration; pH-value; Enzymatic reaction; | 98% |
| With nicotinamide adenine dinucleotide phosphate; isopropyl alcohol In aq. phosphate buffer at 37℃; pH=7; | 93% |
| With potassium borohydride; potassium tert-butylate In isopropyl alcohol at 40℃; for 24h; | 92% |

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; aluminum nickel In water | 98% |

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| Stage #1: C24H36O4 With potassium tert-butylate In tert-butyl alcohol for 0.5h; Stage #2: With palladium 10% on activated carbon; hydrogen In tert-butyl alcohol at 80℃; under 3750.38 Torr; for 12h; Solvent; Reagent/catalyst; Temperature; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: 7-Ketolithocholic acid With potassium tert-butylate; palladium(II) hydroxide; potassium hydroxide In isopropyl alcohol at 20℃; for 0.166667h; Stage #2: With hydrogen In isopropyl alcohol at 40 - 80℃; Reagent/catalyst; Temperature; | A n/a B 95% |
| With sodium tetrahydroborate; sodium hydrogencarbonate In water at 20℃; for 0.5h; var. reducing agents; | A 94% B 2% |
| With potassium In tert-butyl alcohol for 0.5h; Heating; var. reducing agents; | A 6% B 94% |

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 20℃; for 6h; | 94% |

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With potassium borohydride; potassium tert-butylate; hydrogen at 40℃; under 30003 Torr; for 24h; | 93% |
| With potassium borohydride; potassium tert-butylate; hydrogen In isopropyl alcohol at 40℃; under 30003 Torr; for 24h; Autoclave; | 93% |
| With potassium borohydride; potassium tert-butylate; hydrogen In isopropyl alcohol at 40℃; under 30003 Torr; for 24h; | 93% |

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| Stage #1: (E)-7-hydroxy-3-oxo-4,6,22-choladienoic acid ethyl ester In 2-methyltetrahydrofuran at 90℃; under 30003 Torr; for 24h; Autoclave; Stage #2: With sodium t-butanolate In 2-methyltetrahydrofuran; isopropyl alcohol at 90℃; under 30003 Torr; for 24h; Solvent; Reagent/catalyst; Temperature; Autoclave; | 87% |

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| Stage #1: C26H36O4 With hydrogen In 2-methyltetrahydrofuran at 90℃; under 30003 Torr; for 24h; Autoclave; Stage #2: With sodium t-butanolate In 2-methyltetrahydrofuran; isopropyl alcohol at 90℃; for 24h; Solvent; Reagent/catalyst; Temperature; | 87% |

| Conditions | Yield |
|---|---|
| With NAD In methanol; aq. phosphate buffer at 25℃; for 24h; pH=8; | 86% |
| With 7β-hydroxysteroid dehydrogenase; Sepharose-CL6B; TEA; nicotinamide adenine dinucleotide In water at 20℃; for 120h; | 75% |
| Multi-step reaction with 2 steps 1: potassium phosphate, pyruvate, dithiothreitol, NAD / H2O / Ambient temperature; lactic dehydrogenase, 7α-hydroxysteroid dehydrogenase 2: potassium phosphate, glucose, dithiothreitol, NADP / H2O / glucose dehydrogenase, 7β-hydroxysteroid dehydrogenase View Scheme |
-
-
434-13-9
Lithocholic acid

-
A
-
128-13-2
ursodeoxycholic acid

-
B
-
77060-26-5
3-keto-7β-hydroxy-5β-cholan-24-oic acid

| Conditions | Yield |
|---|---|
| With Gibberella zeae VKM F-2600 extract In methanol; aq. phosphate buffer at 29℃; for 122h; pH=7; Microbiological reaction; | A 83% B n/a |
-
-
81873-91-8
3α,7β-dihydroxy-12-oxo-5β-cholane-24-oic acid

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; potassium hydroxide; 2,2'-[1,2-ethanediylbis(oxy)]bisethanol at 110 - 135℃; Wolf-Kishner reduction; | 82% |

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With potassium borohydride; potassium tert-butylate; hydrogen at 40℃; under 30003 Torr; for 24h; | 80% |
| Multi-step reaction with 2 steps 1: sodium hydroxide; water / methanol; tetrahydrofuran / 4 h / Reflux; Inert atmosphere 2: potassium tert-butylate; potassium borohydride; hydrogen / isopropyl alcohol / 24 h / 40 °C / 30003 Torr / Autoclave View Scheme |

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With 1,4-dihydronicotinamide adenine dinucleotide; 7β-hydroxysteroid dehydrogenase; 2-hydroxyethanethiol; ethylenediaminetetraacetic acid In phosphate buffer at 20℃; for 48h; pH=7; | 75% |

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 3α-benzoyloxy-7β-hydroxy-chol-5-en-24-oate With palladium 10% on activated carbon; hydrogen; acetic acid In ethanol under 2844.39 Torr; for 24h; Stage #2: With methanol; sodium hydroxide for 18h; Reflux; | 71% |

| Conditions | Yield |
|---|---|
| With ammonia; lithium In tetrahydrofuran; methanol at -50℃; for 1h; | 68% |
-
-
10452-65-0
methyl 3α-acetoxy-7-oxo-5β-cholan-24-oate

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With sodium In butan-1-ol at 20 - 60℃; for 2.25h; Inert atmosphere; | 62% |

| Conditions | Yield |
|---|---|
| With air; Xanthomonas maltophilia CBS 827.97 In sodium hydroxide at 30℃; for 4h; pH=8; epimerisation; | A 27% B 23% |
| With Xanthomonas maltophilia In water at 30℃; for 24h; pH=8; anaerobic; | A 27% B 23% |

| Conditions | Yield |
|---|---|
| With aluminum isopropoxide; isopropyl alcohol |
-
-
4651-67-6
7-Ketolithocholic acid

-
-
141-52-6
sodium ethanolate

-
A
-
474-25-9
chenodeoxycholic acid

-
B
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| at 200℃; |

-
-
859-97-2
3,7-diketocholanic acid

-
-
555-31-7
aluminum isopropoxide

-
-
67-63-0
isopropyl alcohol

-
A
-
474-25-9
chenodeoxycholic acid

-
B
-
128-13-2
ursodeoxycholic acid

-
-
81857-23-0
methyl 3-O-acetyl-7-O-mesylchenodeoxycholate

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With potassium superoxide; 18-crown-6 ether In dimethyl sulfoxide for 3h; Yield given; |

-
A
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| In water; ethylene glycol at 25℃; Equilibrium constant; decomplexation; |

-
A
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| In water; ethylene glycol at 25℃; Equilibrium constant; decomplexation; |
-
-
7647-01-0
hydrogenchloride

-
-
4651-67-6
7-Ketolithocholic acid

-
-
64-19-7
acetic acid

-
A
-
474-25-9
chenodeoxycholic acid

-
B
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| Hydrogenation; |


| Conditions | Yield |
|---|---|
| With chloylglycine hydrolase In phosphate buffer at 84℃; for 0.0833333h; |
-
-
16564-43-5
glycochenodeoxycholic acid sodium salt

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 100 percent Chromat. / chloylglycine hydrolase; β-mercaptoethanol; EDTA / aq. phosphate buffer / 3 h / 20 °C / pH 8 2: 75 percent / Sepharose-CL6B; 7β-hydroxysteroid dehydrogenase; TEA / NAD(+) / H2O / 120 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 100 percent Chromat. / chloylglycine hydrolase; β-mercaptoethanol; EDTA / aq. phosphate buffer / 0.33 h / 20 °C / pH 8 2: 75 percent / Sepharose-CL6B; 7β-hydroxysteroid dehydrogenase; TEA / NAD(+) / H2O / 120 h / 20 °C View Scheme |
-
-
10538-64-4
methyl 3α,7α-dihydroxy-12-oxo-5β-cholan-24-oate

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 52 percent / acetic acid / 12 h / Ambient temperature 2: 57 percent / NaBH4 / acetic acid / 3 h / Ambient temperature 3: pyridine / benzene 4: pyridine / Ambient temperature 5: KO2/18-crown-6 ether / dimethylsulfoxide / 3 h View Scheme |

| Conditions | Yield |
|---|---|
| With triethylamine; 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride In N,N-dimethyl-formamide at 20℃; for 1h; | 100% |
-
-
67-56-1
methanol

-
-
128-13-2
ursodeoxycholic acid

-
-
10538-55-3
3α,7β-dihydroxy-5β-cholan-24-oic acid methyl ester

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 2.5h; Reflux; | 98% |
| With sulfuric acid at 50℃; for 3h; | 97% |
| With toluene-4-sulfonic acid at 65℃; for 2.5h; | 97% |
-
-
128-13-2
ursodeoxycholic acid

-
-
108-24-7
acetic anhydride

-
-
6533-77-3
(R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-diacetoxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid

| Conditions | Yield |
|---|---|
| In pyridine at 20℃; for 12h; | 98% |
| With dmap In dichloromethane at 20℃; for 1h; Inert atmosphere; | 95% |
| With pyridine at 20℃; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 10 - 20℃; for 2h; Temperature; Concentration; | 97.3% |
-
-
64-18-6
formic acid

-
-
128-13-2
ursodeoxycholic acid

-
-
6058-15-7, 6159-50-8
(R)-4-((3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-bis(formyloxy)-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)pentanoic acid

| Conditions | Yield |
|---|---|
| at 55℃; for 20h; | 96% |
| With perchloric acid | 96% |
| 1) 65 deg C, 4 h, 2) 25 deg C, 20 h; | 95% |
-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With iron(III) chloride; sodium hydroxide In water at 35℃; pH=8; | 95% |

| Conditions | Yield |
|---|---|
| With N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline; sodium hydroxide In water; acetonitrile; tert-butyl alcohol at 20 - 80℃; | 95% |
-
-
128-13-2
ursodeoxycholic acid

-
-
100-39-0
benzyl bromide

-
-
111992-93-9
benzyl 3α,7β-dihydroxy-5β-cholan-24-oate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; for 3h; | 95% |
| With potassium carbonate In acetonitrile at 80℃; for 3h; | 95% |
| Stage #1: ursodeoxycholic acid With potassium hydrogencarbonate In N,N-dimethyl-formamide for 0.333333h; Stage #2: benzyl bromide In N,N-dimethyl-formamide for 48h; | 95% |
| With potassium carbonate In acetonitrile at 80℃; for 3h; | 95% |
| With caesium carbonate In acetonitrile for 4h; Reflux; | 82% |
-
-
128-13-2
ursodeoxycholic acid

-
-
23848-46-6, 130593-75-8
UDC-OH

| Conditions | Yield |
|---|---|
| With samarium diiodide; water; triethylamine In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; | 94% |
| With lithium aluminium tetrahydride | |
| Multi-step reaction with 2 steps 1: thionyl chloride / 3.5 h / 20 °C / Reflux 2: sodium tetrahydroborate; ethanol / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 24h; Ambient temperature; | 93% |
-
-
128-13-2
ursodeoxycholic acid

-
-
6000-43-7
2-aminoethanoic acid hydrochloride

-
-
115488-03-4
glycine ursodeoxycholic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In acetone for 12h; Solvent; Time; Reflux; | 93% |
-
-
128-13-2
ursodeoxycholic acid

-
-
75-36-5
acetyl chloride

-
-
10538-55-3
3α,7β-dihydroxy-5β-cholan-24-oic acid methyl ester

| Conditions | Yield |
|---|---|
| In methanol at 0 - 20℃; for 6h; | 92% |
-
-
128-13-2
ursodeoxycholic acid

-
A
-
88426-32-8
3,7-bis(sulfooxy)ursodeoxycholic acid

-
B
-
59132-32-0, 64520-49-6, 68780-68-7, 68833-02-3, 124815-69-6, 68780-73-4
7-hydroxy-3-(sulfooxy)-(3α,5β,7β)-cholan-24-oic acid

-
C
-
74723-14-1
7-(sulfooxy)ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| With pyridine; sulfur trioxide pyridine complex at 0 - 20℃; for 0.166667h; other temperatures, other times, other reagent ratio; | A 91.9% B 4.1% C 2.6% |
| With pyridine; sulfur trioxide pyridine complex at -5℃; for 0.166667h; Product distribution; other temperatures, other reaction times, other reagent ratio; | A 25% B 31.2% C 25% |
| With pyridine; sulfur trioxide pyridine complex at -5℃; for 0.166667h; other temperatures, other times, other reagent ratio; | A 25% B 31.2% C 25% |
| With pyridine; sulfur trioxide pyridine complex at -5℃; for 0.166667h; other temperatures, other times, other reagent ratio; | A 25% B 31.2% C 25% |

-
-
63147-26-2
trimethyl-sulfo-ammonium betaine

-
-
128-13-2
ursodeoxycholic acid

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 40 - 90℃; | 91.6% |

| Conditions | Yield |
|---|---|
| Stage #1: ursodeoxycholic acid; glycine With N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline; sodium hydroxide In water; acetonitrile; tert-butyl alcohol at 20 - 80℃; Stage #2: With hydrogenchloride In water pH=2; | 91% |
-
-
109-01-3
1-methyl-piperazine

-
-
128-13-2
ursodeoxycholic acid

-
-
86678-72-0
N1<(3α,5β,7β)3,7-dihydroxy-24-oxo-cholan-24-yl>N4 methyl-piperazine

| Conditions | Yield |
|---|---|
| With tributyl-amine; chloroformic acid ethyl ester In 1,4-dioxane 10 deg C, 10 min then rt., 1 h; | 90% |
-
-
128-13-2
ursodeoxycholic acid

-
-
77060-26-5
3-keto-7β-hydroxy-5β-cholan-24-oic acid

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; D-glucose; Pseudomonas paucimobilis; yeast extracxt; peptone In water at 28℃; for 24h; | 90% |
| Multi-step reaction with 4 steps 1: triethylamine; DMAP / ethyl acetate / 10 h / Heating 2: 5percent NaOH / methanol / 1 h 3: Jones' reagent / acetone / 0.08 h 4: 95 percent / 20percent NaOH / 1 h / Heating View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: ursodeoxycholic acid With triethylamine; isobutyl chloroformate In N,N-dimethyl-formamide at -15℃; for 0.25h; Inert atmosphere; Stage #2: arabinosyl cytosine With triethylamine In N,N-dimethyl-formamide at -15 - 20℃; for 0.5h; Inert atmosphere; | 87% |
| Stage #1: ursodeoxycholic acid With triethylamine; isobutyl chloroformate In N,N-dimethyl-formamide at -15℃; for 0.0833333h; Stage #2: arabinosyl cytosine With triethylamine In N,N-dimethyl-formamide at -15 - 20℃; for 2.5h; |

| Conditions | Yield |
|---|---|
| With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 20℃; for 16h; | 87% |
| With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane at 20℃; for 16h; | 87% |
Ursodeoxycholic acid Chemical Properties
Molecule structure of Ursodeoxycholic acid (CAS NO.128-13-2):
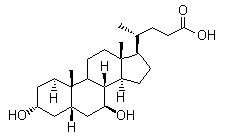
IUPAC Name: (4R)-4-[(3R,5S,7S,8R,9S,10S,13R,14S,17R)-3,7-Dihydroxy-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl]pentanoic acid
Molecular Weight: 392.572 g/mol
Molecular Formula: C24H40O4
Density: 1.128 g/cm3
Melting Point: 203-204 °C(lit.)
Boiling Point: 547.1 °C at 760 mmHg
Flash Point: 298.8 °C
Index of Refraction: 1.543
Molar Refractivity: 109.65 cm3
Molar Volume: 347.8 cm3
Surface Tension: 46 dyne/cm
Enthalpy of Vaporization: 95.01 kJ/mol
Vapour Pressure: 2.98E-14 mmHg at 25 °C
Solubility Ethanol: 50 mg/mL, clear
Water Solubility: practically insoluble
XLogP3-AA: 4.9
H-Bond Donor: 3
H-Bond Acceptor: 4
Rotatable Bond Count: 4
Exact Mass: 392.29266
MonoIsotopic Mass: 392.29266
Topological Polar Surface Area: 77.8
Heavy Atom Count: 28
Canonical SMILES: CC(CCC(=O)O)C1CCC2C1(CCC3C2C(CC4C3(CCC(C4)O)C)O)C
Isomeric SMILES: C[C@H](CCC(=O)O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2[C@H](C[C@H]4C@@]3(CC[C@H](C4)O)C)O)C
InChI: InChI=1S/C24H40O4/c1-14(4-7-21(27)28)17-5-6-18-22-19(9-11-24(17,18)3)23(2)10-8-16(25)12-15(23)13-20(22)26/h14-20,22,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15+,16-,17-,18+,19+,20+,22+,23+,24-/m1/s1
InChIKey: RUDATBOHQWOJDD-UZVSRGJWSA-N
EINECS: 204-879-3
Product Categories: Bile Acids; Biochemistry; Steroids; Intermediates & Fine Chemicals; Pharmaceuticals; API's
Ursodeoxycholic acid Uses
Ursodeoxycholic acid (CAS NO.128-13-2) can be used as an anticholelithogenic.
Ursodeoxycholic acid Production
Ursodeoxycholic acid is found in large quantities in bear bile.
Ursodeoxycholic acid Toxicity Data With Reference
| 1. | mmo-sat 40 mg/L | MUREAV Mutation Research. 158 (1985),45. | ||
| 2. | orl-rat TDLo:11,900 mg/kg (4-20D preg):TER | AIPTAK Archives Internationales de Pharmacodynamie et de Therapie. 246 (1980),149. | ||
| 3. | orl-rat LD50:4600 mg/kg | BCFAAI Bollettino Chimico Farmaceutico. 126 (1987),282. | ||
| 4. | ipr-rat LD50:890 mg/kg | NIIRDN “Drugs in Japan. Ethical Drugs, 6th Edition 1982“ Edited by Japan Pharmaceutical Information Center. 6 (1982),95. | ||
| 5. | ivn-rat LD50:310 mg/kg | NIIRDN “Drugs in Japan. Ethical Drugs, 6th Edition 1982“ Edited by Japan Pharmaceutical Information Center. 6 (1982),95. | ||
| 6. | ivn-mus LD50:240 mg/kg | NIIRDN “Drugs in Japan. Ethical Drugs, 6th Edition 1982“ Edited by Japan Pharmaceutical Information Center. 6 (1982),95. |
Ursodeoxycholic acid Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 24/25-36-26
S24/25:Avoid contact with skin and eyes.
S36:Wear suitable protective clothing.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
WGK Germany: 2
RTECS: FZ2000000
F: 10
Poison by intraperitoneal and intravenous routes. Experimental teratogenic and reproductive effects. Mutation data reported. Stimulates the flow of bile to the duodenum (a cholagogic). When heated to decomposition it emits acrid smoke and irritating fumes.
Ursodeoxycholic acid Specification
Ursodeoxycholic acid (CAS NO.128-13-2) is also named as 17-beta-(1-Methyl-3-carboxypropyl)etiocholane-3-alpha,7-beta-diol ; 3,7-Dihydroxycholan-24-oic acid ; 3-alpha,7-beta-Dihydroxy-5-beta-cholanoic acid ; 3-alpha,7-beta-Dihydroxycholanic acid ; 3-alpha,7-beta-Dioxycholanic acid ; 3alpha,7beta-Dihydroxy-5beta-cholan-24-oic acid ; 4-10-00-01604 (Beilstein Handbook Reference) ; 7-beta-Hydroxylithocholic acid ; Actigall ; Arsacol ; BRN 3219888 ; CCRIS 5502 ; Cholit-ursan ; Delursan ; Destolit ; Deursil ; Litursol ; Lyeton ; NSC 657950 ; NSC 683769 ; Peptarom ; Solutrat ; UNII-724L30Y2QR ; UrSO ; Ursacol ; Urso 250 ; Urso DS ; Urso Forte ; Ursobilin ; Ursochol ; Ursocholic acid, deoxy- ; Ursodamor ; Ursodeoxycholate ; Ursodiol ; Ursofalk ; Ursolvan . Ursodeoxycholic acid (CAS NO.128-13-2) is white crystalline powder.
Related Products
- Ursodeoxycholic acid
- Ursodeoxycholic-2,2,4,4-D4 acid
- 128140-58-9
- 128140-82-9
- 128143-89-5
- 128196-01-0
- 128196-02-1
- 128-20-1
- 128208-00-4
- 128-21-2
- 128221-48-7
- 128227-97-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View