-
Name
beta-Alanine
- EINECS 203-536-5
- CAS No. 107-95-9
- Article Data231
- CAS DataBase
- Density 1.166 g/cm3
- Solubility water: 1 M at 20 °C, clear, colorless
- Melting Point 202 °C (dec.)(lit.)
- Formula C3H7NO2
- Boiling Point 237.1 °C at 760 mmHg
- Molecular Weight 89.0941
- Flash Point 97.2 °C
- Transport Information
- Appearance white crystalline powder
- Safety 24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms β-Aminopropionic acid;omega-Aminopropionic acid;beta-Alanine (6CI,8CI,9CI);Propanoic acid, 3-amino-;Alanine, beta-;beta-aminopropionic acid;3-Aminopropionsaeure;a-Alanine;Alanine (beta-);H-β-Alanine;H-β-Ala-OH;3-amino-;.beta.-Aminopropionic acid;3-Aminopropionic acid;beta-Aminopropionsaeure;2-Carboxyethylamine;3-aminopropanoate;Abufene;3-azaniumylpropanoate;3-Aminopropanoic acid;
- PSA 63.32000
- LogP 0.12010
Synthetic route
-
-
137-08-6, 867-80-1, 6381-62-0, 6381-63-1, 15773-29-2, 64789-49-7, 122484-60-0, 131346-21-9
calcium-N-(2,4-dihydroxy-3,3-dimethylbutyryl)-β-alaninate

-
A
-
79-50-5
pantolactone

-
B
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 80℃; for 1.75h; Rate constant; | A 100% B 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 80℃; for 1.25h; Rate constant; | A 100% B 100% |

| Conditions | Yield |
|---|---|
| With water at 170℃; for 0.05h; Microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| In water at 35℃; for 6h; Temperature; Enzymatic reaction; | 98.4% |
| With water; sodium hydroxide at 100 - 110℃; under 37503.8 Torr; for 0.025h; Concentration; Pressure; Temperature; Flow reactor; | 98% |
| Stage #1: 2-cyanoethylamine With hydrogenchloride In water Stage #2: In water at 40℃; for 8h; pH=7; Enzymatic reaction; Stage #3: With hydrogenchloride In water at 40℃; for 8h; pH=6; Kinetics; Enzymatic reaction; | 90% |

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In methanol for 0.333333h; Ambient temperature; | 98% |
| With hydrogen at 100℃; under 7500.75 Torr; for 12h; Sealed tube; Autoclave; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2-cyanoacetate With hydrogen In ethanol at 100℃; under 7500.75 Torr; for 5h; Autoclave; Stage #2: With water In ethanol | 94% |
| With sulfuric acid; acetic acid; platinum Hydrogenation.unter Druck und Hydrolyse der Reaktionsprodukts; | |
| With hydrogenchloride; palladium Hydrogenation.Hydrolyse des Reaktionsprodukts; |

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 2-cyanoacetate With hydrogen In ethanol at 80℃; under 3750.38 Torr; for 10h; Autoclave; Stage #2: With water In ethanol | 92% |
| With hydrogenchloride; palladium Hydrogenation.Hydrolyse des Reaktionsprodukts; | |
| With ethanol; nickel Hydrogenation.Hydrolyse des Reaktionsprodukts; |
-
-
125506-43-6
N-Diphenylmethylene-β-alanine benzyl ester

-
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol under 760 Torr; for 14h; Ambient temperature; | 90% |
-
-
95503-55-2
N-benzylisoxazolidin-5-one

-
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In 1,4-dioxane; water at 60℃; for 16h; | 85% |

| Conditions | Yield |
|---|---|
| With aspartate-α-decarboxylase; 3-methylaspartate ammonia lyase; ammonium chloride; magnesium chloride In aq. buffer at 25℃; for 24h; pH=8; Enzymatic reaction; | 85% |

| Conditions | Yield |
|---|---|
| With lithium hydroxide; [cis-β-Co(1,4,7,10-tetraazadecane)Cl2]Cl In water at 20 - 45℃; for 2.25h; pH=7.7 - 8; | A 84% B n/a |

| Conditions | Yield |
|---|---|
| With L-aspartase from E.coli; L-aspartate-α-decarboxylase from Corynebacterium glutamicum; ammonia at 37℃; for 12h; pH=7; Reagent/catalyst; Enzymatic reaction; | 82% |
-
-
16856-13-6
(+)-β-methyl-L-aspartate hydrochloride

-
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| In various solvent(s) from 160 up to 180 deg C over 90 min; | 62% |
-
-
64-18-6
formic acid

-
-
107-11-9
1-amino-2-propene

-
A
-
107-10-8
propylamine

-
B
-
10569-72-9
DL-3-aminoisobutyric acid

-
C
-
56-40-6
glycine

-
D
-
107-95-9
3-amino propanoic acid

-
E
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| With hydrogen; oxygen In water for 3h; Product distribution; various unsaturated amines; investigation of the direct carboxylation of C=C bond, the effect of formic acid concentration as well as the flame composition on product(s); radical mechanism is proposed; | A n/a B 38% C n/a D n/a E 5% |

-
-
64-18-6
formic acid

-
-
107-11-9
1-amino-2-propene

-
A
-
10569-72-9
DL-3-aminoisobutyric acid

-
B
-
56-40-6
glycine

-
C
-
107-95-9
3-amino propanoic acid

-
D
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| With hydrogen; oxygen In water for 3h; Further byproducts given; | A 38% B n/a C n/a D 5% |


-
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| With adamantane; ammonium chloride In solid high-pressure extrusion (15-30 kbar); | 10% |
| at -173.1℃; under 75006 Torr; | 3.5 % Chromat. |
-
-
107-10-8
propylamine

-
-
64-18-6
formic acid

-
A
-
10569-72-9
DL-3-aminoisobutyric acid

-
B
-
56-40-6
glycine

-
C
-
2835-81-6
2-aminobutanoic acid

-
D
-
107-95-9
3-amino propanoic acid

-
E
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; Product distribution; contact glow discharge electrolysis; variation of pH, effect of time; | A 9.8% B 0.2% C 0.9% D 3.4% E 8.1% |

-
-
107-10-8
propylamine

-
-
64-18-6
formic acid

-
A
-
10569-72-9
DL-3-aminoisobutyric acid

-
B
-
2835-81-6
2-aminobutanoic acid

-
C
-
107-95-9
3-amino propanoic acid

-
D
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; contact glow discharge electrolysis (500-600 V, 45 mA); Further byproducts given; | A 9.8% B 0.9% C 3.4% D 8.1% |

-
-
117614-90-1
(2S,3'S,5'R,6'R)-6'-(3-Carboxy-propyl)-[2,3']bipiperidinyl-5'-carboxylic acid

-
A
-
3105-95-1
pipecolinic acid

-
B
-
107-95-9
3-amino propanoic acid

-
C
-
56-12-2
4-amino-n-butyric acid

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide In sulfuric acid at 100℃; for 6h; | A 9% B n/a C n/a |

-
-
64-18-6
formic acid

-
-
75-04-7
ethylamine

-
A
-
302-84-1
serin

-
B
-
56-40-6
glycine

-
C
-
302-72-7
rac-Ala-OH

-
D
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; contact glow discharge electrolysis (500-600 V, 45 mA); | A 0.1% B 0.1% C 1.5% D 3.1% |

-
-
141-53-7
sodium formate

-
-
75-04-7
ethylamine

-
A
-
302-84-1
serin

-
B
-
56-40-6
glycine

-
C
-
302-72-7
rac-Ala-OH

-
D
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| In water at 10 - 20℃; for 1h; contact glow discharge electrolysis (500-600 V, 45 mA); | A 1% B 0.3% C 3.1% D 0.6% |


| Conditions | Yield |
|---|---|
| With chromium(III) oxide; sulfuric acid |

| Conditions | Yield |
|---|---|
| With ammonia; water at 20℃; | |
| With ammonia; water at 70℃; |

| Conditions | Yield |
|---|---|
| With ammonia; water |

| Conditions | Yield |
|---|---|
| With alkaline NaOCl at 60 - 70℃; | |
| With potassium hydroxide; bromine at 50 - 60℃; | |
| With potassium hydroxide; bromine at 55 - 60℃; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With ammonia; water; diphenylamine at 200℃; | |
| With ammonia; water; diphenylamine at 200℃; |

| Conditions | Yield |
|---|---|
| at 170℃; for 6h; | 100% |
| at 185 - 200℃; for 0.25h; | 98.4% |
| With triethylamine In toluene for 2h; Reagent/catalyst; Reflux; | 97.7% |

| Conditions | Yield |
|---|---|
| In methanol at 60℃; for 6h; | 100% |
| In methanol for 6h; Reflux; | 100% |
| In chloroform at 60℃; for 5h; Concentration; Solvent; Temperature; | 95.4% |

| Conditions | Yield |
|---|---|
| Stage #1: methanol With thionyl chloride for 1h; Cooling with ice; Stage #2: 3-amino propanoic acid at 20 - 66℃; for 6.5h; | 100% |
| With thionyl chloride for 3h; Reflux; | 100% |
| With thionyl chloride at 0 - 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 20 - 50℃; | 100% |
| With hydrogenchloride | |
| With thionyl chloride |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 20℃; for 2h; Product distribution / selectivity; Heating / reflux; | 100% |
| With thionyl chloride Heating / reflux; | 79.08% |
| With hydrogenchloride |
-
-
98-03-3
thiophene-2-carbaldehyde

-
-
107-95-9
3-amino propanoic acid

-
-
101153-25-7
3-(N-thiophene-2-aldimino)propanoic acid

| Conditions | Yield |
|---|---|
| piperidine In ethanol for 2h; Heating; | 100% |
-
-
1072-83-9
2-Acetylpyrrole

-
-
107-95-9
3-amino propanoic acid

-
-
131526-01-7
(pyrrole-2-acetylidene-amino)propionoc acid

| Conditions | Yield |
|---|---|
| With piperidine In ethanol Heating; | 100% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
107-95-9
3-amino propanoic acid

-
-
3303-84-2
3-(tert-butyloxycarbonylamino)propionic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; tert-butyl alcohol at 20℃; | 100% |
| With sodium hydroxide In 1,4-dioxane; water at 0℃; | 100% |
| With sodium hydroxide In tetrahydrofuran at 20℃; for 16h; | 100% |
-
-
100-14-1
4-nitrobenzyl chloride

-
-
107-95-9
3-amino propanoic acid

-
-
294201-15-3
N-4-nitrobenzyl-β-alanine

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 20h; | 100% |
-
-
2937-50-0
Allyl chloroformate

-
-
107-95-9
3-amino propanoic acid

-
-
111695-91-1
N-(allyloxycarbonyl)-3-aminopropionic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran at 20℃; for 1h; Acylation; | 100% |
| With sodium carbonate In 1,4-dioxane; water at 20℃; for 48h; | 97% |
| With sodium hydroxide In tetrahydrofuran; 1,4-dioxane at 0 - 20℃; for 3h; | 52% |
| With sodium hydroxide In water at 5℃; | 46% |
| Stage #1: 3-amino propanoic acid With sodium carbonate In water Stage #2: Allyl chloroformate In water at 0 - 20℃; for 32h; pH=> 9; Stage #3: With hydrogenchloride In water at 0℃; pH=1 - 2; |
-
-
122641-57-0
2,3-Bis(2-methylbenzothiophen-3-yl)maleic anhydride

-
-
107-95-9
3-amino propanoic acid

-
-
1034806-12-6
3,4-bis(2-methylbenzo[b]thiophen-3-yl)-1-(propanoic acid-3-yl)-1H-pyrrole-2,5-dione

| Conditions | Yield |
|---|---|
| at 150℃; for 1h; | 100% |
-
-
135544-68-2
N-(allyloxycarbonyloxy)succinimide

-
-
107-95-9
3-amino propanoic acid

-
-
111695-91-1
N-(allyloxycarbonyl)-3-aminopropionic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water pH=10; Cooling with ice; | 100% |
-
-
2052-49-5
tetra(n-butyl)ammonium hydroxide

-
-
107-95-9
3-amino propanoic acid

-
-
104761-05-9
β-alanine tetrabutylammonium salt

| Conditions | Yield |
|---|---|
| In methanol; water at 0 - 20℃; for 15h; | 100% |
| In methanol; water at 22℃; for 15h; | 80% |
-
-
32276-56-5
3-carboxybenzoyl chloride

-
-
107-95-9
3-amino propanoic acid

-
-
125038-74-6
isophthaloylbis-β-alanine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; toluene at 10℃; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-amino propanoic acid With lithium hydroxide In water at 55℃; for 1.08333h; pH=9 - 9.5; Stage #2: chloroacetic acid In water at 45 - 50℃; for 4.5h; Stage #3: With potassium iodide In water at 92℃; for 8.5h; Reagent/catalyst; | 99.9% |
-
-
85-44-9
phthalic anhydride

-
-
112-42-5
undecyl alcohol

-
-
113170-57-3
tin (II) oxalate

-
-
107-95-9
3-amino propanoic acid

| Conditions | Yield |
|---|---|
| 99.6% |

-
-
501-53-1
benzyl chloroformate

-
-
107-95-9
3-amino propanoic acid

-
-
2304-94-1
3-(benzyloxycarbonylamino)propanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 0 - 20℃; | 99% |
| With sodium carbonate In 1,4-dioxane; water at 20℃; | 96% |
| With sodium carbonate In 1,4-dioxane; water at 0 - 20℃; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: formic acid; 3-amino propanoic acid at 20 - 55℃; Stage #2: With acetic anhydride at 20 - 55℃; for 2h; Stage #3: With water Heating; Reduced pressure; | 99% |
| Stage #1: formic acid With acetic anhydride at 45℃; for 1h; Stage #2: 3-amino propanoic acid at 20℃; for 24h; | 93% |
| With acetic anhydride at 50℃; for 2h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol 1) 0 deg C, 1 h, 2) r.t., 4 h; | 99% |
| In acetonitrile at 35 - 40℃; for 6h; | 87% |
| With sodium hydroxide In water for 1h; Cooling with ice; | 68.8% |
-
-
107-95-9
3-amino propanoic acid

-
-
40391-99-9
pamidronate

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; phosphorous acid; phosphorus trichloride at 65 - 70℃; Inert atmosphere; | 99% |
| Stage #1: 3-amino propanoic acid With phosphorus trichloride In methanesulfonic acid at 80 - 85℃; Stage #2: With water In methanesulfonic acid at 105℃; for 4h; | 85% |
| With phosphonic Acid; phosphorus trichloride In chlorobenzene at 100℃; for 3h; Inert atmosphere; | 82% |

| Conditions | Yield |
|---|---|
| Heating; | 99% |
-
-
407-25-0
trifluoroacetic anhydride

-
-
107-95-9
3-amino propanoic acid

-
-
50632-82-1
3-(2,2,2-trifluoroacetamido)propanoic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran from -5 deg C up to r.t. over 1 h.; | 99% |
-
-
82911-69-1
N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide

-
-
107-95-9
3-amino propanoic acid

-
-
35737-10-1
N-Fmoc-β-alanine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; acetonitrile at 20℃; | 99% |
| With triethylamine In 1,4-dioxane; water at 20℃; for 0.5h; | 95% |
| With triethylamine In water for 0.5h; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 25℃; for 1h; | 99% |
| With hydrogenchloride | 99% |

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane for 1h; Heating; | 99% |
-
-
14223-48-4
p-chlorophenyl isoselenocyanate

-
-
107-95-9
3-amino propanoic acid

-
-
1310370-52-5
C10H11ClN2O2Se

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 100℃; for 15h; | 99% |
beta-Alanine Specification
1. Introduction of β-Alanine
For being one kind of powder, β-Alanine has its IUPAC name of 3-aminopropanoic acid. It is an amino acid formed in vivo by the degradation of dihydrouracil and carnosine. It is a component of the naturally occurring peptides carnosine and anserine and also of pantothenic acid (vitamin B5) which itself is a component of coenzyme A. Besides, it should be stored in sealed container, and put in a cool and dry place, away from oxidant. As to the application of β-Alanine, it can be used as electroplating corrosion inhibitors and biochemical reagents.
2. Properties of β-Alanine
Physical properties about this chemical are: (1)ACD/LogP: -0.86; (2)ACD/LogD (pH 5.5): -3.36; (3)ACD/LogD (pH 7.4): -3.36; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 3; (9)#H bond donors: 3; (10)#Freely Rotating Bonds: 3; (11)Polar Surface Area: 29.54 Å2; (12)Index of Refraction: 1.463; (13)Molar Refractivity: 21.04 cm3; (14)Molar Volume: 76.3 cm3; (15)Polarizability: 8.34 ×10-24cm3; (16)Surface Tension: 49.3 dyne/cm; (17)Density: 1.166 g/cm3; (18)Flash Point: 97.2 °C; (19)Enthalpy of Vaporization: 52.2 kJ/mol; (20)Boiling Point: 237.1 °C at 760 mmHg; (21)Vapour Pressure: 0.0156 mmHg at 25°C.
3. Structure Descriptors of β-Alanine
You could convert the following datas into the molecular structure:
(1). InChI: InChI=1S/C3H7NO2/c4-2-1-3(5)6/h1-2,4H2,(H,5,6)
(2). InChIKey: InChIKey=UCMIRNVEIXFBKS-UHFFFAOYSA-N
(3). Smiles: C(C(O)=O)CN
4. Safety Information of β-Alanine
Hazard Codes: Xi
Risk Statements: 36/37/38
Safety Statements: 24/25-36-26
WGK Germany: 3
RTECS UA2369200
HS Code 29224920
5. Preparation of β-Alanine
Preparation of β-Alanine: it can be prepared by β-aminopropylnitrile through hydrolysis. In addition, it can be prepared by 3-nitro-propionic acid. This reaction will need reagent anhydrous ammonium formate, catalyst 10 percent Pd-C and solvent methanol. The reaction time is 20 minutes with ambient temperature. The yield is about 98%.
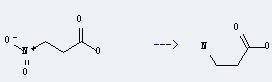
6. Uses of β-Alanine
Uses of β-Alanine: it is used as a raw material to synthesize calcium pantothenate. In addition, it can be used to get azetidin-2-one. This reaction will need reagents methanesulfonyl chloride and NaHCO3, and solvent acetonitrile. The yield is about 80% at the reaction temperature of 80 °C.
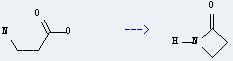
Related Products
- beta-Alanine
- beta-Alanine benzyl ester-p-toluenesulfonate
- beta-Alanine, L-leucyl-
- beta-Alanine, N-beta-alanyl-
- beta-Alanine, N-butyl-N-nitroso-
- beta-Alanine, N-ethyl-N-phenyl-,methyl ester
- 107-96-0
- 1079-66-9
- 107-97-1
- 107971-01-7
- 107978-77-8
- 107979-42-0
- 107-98-2
- 107983-78-8
- 107990-50-1
- 107991-51-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View