-
Name
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one
- EINECS 1308068-626-2
- CAS No. 196597-78-1
- Article Data20
- CAS DataBase
- Density 1.289 g/cm3
- Solubility
- Melting Point 149-151 °C
- Formula C11H10O2
- Boiling Point 334.156 °C at 760 mmHg
- Molecular Weight 174.199
- Flash Point 165.595 °C
- Transport Information
- Appearance White solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 6,7-Dihydro-1H-indeno[5,4-b]furan-8(2H)-one;
- PSA 26.30000
- LogP 1.75040
Synthetic route
-
-
1205098-83-4
1-(2,3-dihydrobenzofuran-4-yl)-2-propen-1-one

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate) In 1,2-dichloro-ethane at 10 - 20℃; for 16h; Solvent; Reagent/catalyst; | 96.3% |
| With sulfuric acid In pentane at 67℃; Product distribution / selectivity; Inert atmosphere; | |
| With sulfuric acid In water; pentane at 67℃; for 0.5h; Inert atmosphere; |
-
-
196597-77-0
4,5-dibromo-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With hydrogen; acetic acid; 10percent Pd/C/H2O at 20℃; for 1h; | 89% |
| With hydrogen; sodium acetate; 5% palladium over charcoal In water; acetic acid at 20℃; | 89% |
| With hydrogen; sodium acetate; palladium 10% on activated carbon In methanol at 40℃; under 2175.22 - 3675.37 Torr; for 8h; | 88.6% |
-
-
196597-77-0
4,5-dibromo-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one

-
A
-
1092507-06-6
C11H12O

-
B
-
1092507-07-7
2,6,7,8-tetrahydro-1H-indeno[5,4-b]furan-8-ol

-
C
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With hydrogen; sodium acetate; acetic acid; palladium 10% on activated carbon under 1471.14 - 2206.72 Torr; | A n/a B n/a C 85% |

-
-
15480-25-8
6,7-dihydro-8H-indeno-[5,4-b]furan-8-one

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With hydrogen; 5% Pd(II)/C(eggshell); triethylamine In tetrahydrofuran; ethanol at 35℃; under 4500.45 - 5250.53 Torr; for 24h; | 62% |
-
-
1198465-69-8
4-tert-butyl-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; aluminum (III) chloride In benzene | 20% |
-
-
496-16-2
2,3-Dihydrobenzofuran

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: 92 percent / TiCl4 / CH2Cl2 / 1 h 2.1: NaH / tetrahydrofuran / 0.33 h / 20 °C 2.2: 88 percent / tetrahydrofuran / 1 h / 20 °C 3.1: 99 percent / H2 / 10percent Pd/C/H2O / ethanol / 2 h / 20 °C 4.1: 97 percent / NaOAc; AcOH; Br2 / 1 h / 20 °C 5.1: 53 percent / AcOH; Fe; Br2 / 5 h / 50 °C 6.1: 93 percent / aq. KOH / ethanol / 1 h / 90 °C 7.1: SOCl2 / 75 °C 8.1: AlCl3 / 1,2-dichloro-ethane / 20 °C 9.1: 89 percent / AcOH; H2 / 10percent Pd/C/H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 9 steps 1.1: 86 percent / POCl3 / 12 h / 80 - 85 °C 2.1: NaH / tetrahydrofuran / 0.33 h / 20 °C 2.2: 88 percent / tetrahydrofuran / 1 h / 20 °C 3.1: 99 percent / H2 / 10percent Pd/C/H2O / ethanol / 2 h / 20 °C 4.1: 97 percent / NaOAc; AcOH; Br2 / 1 h / 20 °C 5.1: 53 percent / AcOH; Fe; Br2 / 5 h / 50 °C 6.1: 93 percent / aq. KOH / ethanol / 1 h / 90 °C 7.1: SOCl2 / 75 °C 8.1: AlCl3 / 1,2-dichloro-ethane / 20 °C 9.1: 89 percent / AcOH; H2 / 10percent Pd/C/H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: trichlorophosphate / 5.17 h / 30 - 98 °C 2.1: sodium methylate / toluene / 0.25 h / 0 - 30 °C 3.1: hydrogen / nickel / methanol / 4 h / 50 °C 4.1: bromine; sodium acetate; acetic acid / 14 h / 20 - 28 °C 5.1: thionyl chloride / N,N-dimethyl-formamide / 1,2-dichloro-benzene / 2 h / 28 - 60 °C 5.2: 1 h / -5 - 5 °C 6.1: hydrogen; sodium acetate / raney chloride / methanol / 50 °C View Scheme |
-
-
55745-70-5
2,3-dihydro-benzofuran-5-carbaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: NaH / tetrahydrofuran / 0.33 h / 20 °C 1.2: 88 percent / tetrahydrofuran / 1 h / 20 °C 2.1: 99 percent / H2 / 10percent Pd/C/H2O / ethanol / 2 h / 20 °C 3.1: 97 percent / NaOAc; AcOH; Br2 / 1 h / 20 °C 4.1: 53 percent / AcOH; Fe; Br2 / 5 h / 50 °C 5.1: 93 percent / aq. KOH / ethanol / 1 h / 90 °C 6.1: SOCl2 / 75 °C 7.1: AlCl3 / 1,2-dichloro-ethane / 20 °C 8.1: 89 percent / AcOH; H2 / 10percent Pd/C/H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: sodium methylate / toluene / 0.25 h / 0 - 30 °C 2.1: hydrogen / nickel / methanol / 4 h / 50 °C 3.1: bromine; sodium acetate; acetic acid / 14 h / 20 - 28 °C 4.1: thionyl chloride / N,N-dimethyl-formamide / 1,2-dichloro-benzene / 2 h / 28 - 60 °C 4.2: 1 h / -5 - 5 °C 5.1: hydrogen; sodium acetate / raney chloride / methanol / 50 °C View Scheme | |
| Multi-step reaction with 6 steps 1.1: piperidine; pyridine / 6 h / 120 °C 2.1: thionyl chloride / 0.67 h / 26 - 73 °C / Heating / reflux 3.1: hydrogen / nickel / methanol / 4 h / 50 °C 4.1: bromine; sodium acetate; acetic acid / 14 h / 20 - 28 °C 5.1: thionyl chloride / N,N-dimethyl-formamide / 1,2-dichloro-benzene / 2 h / 28 - 60 °C 5.2: 1 h / -5 - 5 °C 6.1: hydrogen; sodium acetate / raney chloride / methanol / 50 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: piperidine; pyridine; acetic acid / 4 - 5 h / 100 °C 1.2: 2 - 3 h / 20 °C 2.1: sodium hydroxide; hydrogen; ammonium formate / palladium 10% on activated carbon / water / 5 - 6 h / 20 °C 3.1: bromine; sodium acetate; acetic acid / 2 - 3 h / 0 - 45 °C 4.1: thionyl chloride / N,N-dimethyl-formamide / 1 - 2 h / 20 °C 4.2: 1 - 2 h / 0 - 5 °C 5.1: hydrogen; sodium acetate; acetic acid / palladium 10% on activated carbon / 1471.14 - 2206.72 Torr View Scheme | |
| Multi-step reaction with 6 steps 1: sodium t-butanolate / toluene / 1 h 2: acetic acid; hydrogen; 5%-palladium/activated carbon / 1 h / 50 °C / 1470.15 - 2205.22 Torr 3: acetic acid; sodium acetate; bromine / 6 h / 20 °C / Cooling 4: thionyl chloride / 1,2-dichloro-benzene; N,N-dimethyl-formamide / 42 °C 5: aluminum (III) chloride / 0.5 h / Cooling with ice 6: hydrogen; sodium acetate; palladium 10% on activated carbon / methanol / 2 h / 40 °C / 2205.22 - 3675.37 Torr View Scheme |
-
-
196597-65-6
ethyl (E)-3-(2,3-dihydrobenzofuran-5-yl)-2-propenoate

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 99 percent / H2 / 10percent Pd/C/H2O / ethanol / 2 h / 20 °C 2: 97 percent / NaOAc; AcOH; Br2 / 1 h / 20 °C 3: 53 percent / AcOH; Fe; Br2 / 5 h / 50 °C 4: 93 percent / aq. KOH / ethanol / 1 h / 90 °C 5: SOCl2 / 75 °C 6: AlCl3 / 1,2-dichloro-ethane / 20 °C 7: 89 percent / AcOH; H2 / 10percent Pd/C/H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 5 steps 1: acetic acid; hydrogen; 5%-palladium/activated carbon / 1 h / 50 °C / 1470.15 - 2205.22 Torr 2: acetic acid; sodium acetate; bromine / 6 h / 20 °C / Cooling 3: thionyl chloride / 1,2-dichloro-benzene; N,N-dimethyl-formamide / 42 °C 4: aluminum (III) chloride / 0.5 h / Cooling with ice 5: hydrogen; sodium acetate; palladium 10% on activated carbon / methanol / 2 h / 40 °C / 2205.22 - 3675.37 Torr View Scheme |
-
-
196597-66-7
ethyl 3-(2,3-dihydro-1-benzofuran-5-yl)propanoate

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 97 percent / NaOAc; AcOH; Br2 / 1 h / 20 °C 2: 53 percent / AcOH; Fe; Br2 / 5 h / 50 °C 3: 93 percent / aq. KOH / ethanol / 1 h / 90 °C 4: SOCl2 / 75 °C 5: AlCl3 / 1,2-dichloro-ethane / 20 °C 6: 89 percent / AcOH; H2 / 10percent Pd/C/H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: bromine; sodium acetate; acetic acid / 14 h / 20 - 28 °C 2.1: thionyl chloride / N,N-dimethyl-formamide / 1,2-dichloro-benzene / 2 h / 28 - 60 °C 2.2: 1 h / -5 - 5 °C 3.1: hydrogen; sodium acetate / raney chloride / methanol / 50 °C View Scheme | |
| Multi-step reaction with 4 steps 1: acetic acid; sodium acetate; bromine / 6 h / 20 °C / Cooling 2: thionyl chloride / 1,2-dichloro-benzene; N,N-dimethyl-formamide / 42 °C 3: aluminum (III) chloride / 0.5 h / Cooling with ice 4: hydrogen; sodium acetate; palladium 10% on activated carbon / methanol / 2 h / 40 °C / 2205.22 - 3675.37 Torr View Scheme |
-
-
196597-67-8
ethyl 3-(7-bromo-2,3-dihydro-1-benzofuran-5-yl)propanoate

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 53 percent / AcOH; Fe; Br2 / 5 h / 50 °C 2: 93 percent / aq. KOH / ethanol / 1 h / 90 °C 3: SOCl2 / 75 °C 4: AlCl3 / 1,2-dichloro-ethane / 20 °C 5: 89 percent / AcOH; H2 / 10percent Pd/C/H2O / 1 h / 20 °C View Scheme |
-
-
227179-21-7
3-(6,7-dibromo-2,3-dihydro-benzofuran-5-yl)-propionyl chloride

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: AlCl3 / 1,2-dichloro-ethane / 20 °C 2: 89 percent / AcOH; H2 / 10percent Pd/C/H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: aluminum (III) chloride / 0.5 h / Cooling with ice 2: hydrogen; sodium acetate; palladium 10% on activated carbon / methanol / 2 h / 40 °C / 2205.22 - 3675.37 Torr View Scheme |
-
-
196597-76-9
ethyl 3-(6,7-dibromo-2,3-dihydrobenzofuran-5-yl) propionic acid

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: SOCl2 / 75 °C 2: AlCl3 / 1,2-dichloro-ethane / 20 °C 3: 89 percent / AcOH; H2 / 10percent Pd/C/H2O / 1 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: thionyl chloride / N,N-dimethyl-formamide / 1,2-dichloro-benzene / 2 h / 28 - 60 °C 1.2: 1 h / -5 - 5 °C 2.1: hydrogen; sodium acetate / raney chloride / methanol / 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: thionyl chloride / N,N-dimethyl-formamide / 1 - 2 h / 20 °C 1.2: 1 - 2 h / 0 - 5 °C 2.1: hydrogen; sodium acetate; acetic acid / palladium 10% on activated carbon / 1471.14 - 2206.72 Torr View Scheme | |
| Multi-step reaction with 3 steps 1: thionyl chloride / 1,2-dichloro-benzene; N,N-dimethyl-formamide / 42 °C 2: aluminum (III) chloride / 0.5 h / Cooling with ice 3: hydrogen; sodium acetate; palladium 10% on activated carbon / methanol / 2 h / 40 °C / 2205.22 - 3675.37 Torr View Scheme |
-
-
196597-75-8
ethyl 3-(6,7-dibromo-2,3-dihydro-1-benzofuran-5-yl)propanoate

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 93 percent / aq. KOH / ethanol / 1 h / 90 °C 2: SOCl2 / 75 °C 3: AlCl3 / 1,2-dichloro-ethane / 20 °C 4: 89 percent / AcOH; H2 / 10percent Pd/C/H2O / 1 h / 20 °C View Scheme |
-
-
1198465-72-3
6-hydroxy-7-(2-mesyloxyethyl)indan-1-one

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate for 3h; Reflux; | 4.47 g |
-
-
50-00-0
formaldehyd

-
-
1205098-82-3
1-(dihydrobenzofuran-4-yl)ethanone

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Stage #1: formaldehyd; 1-(dihydrobenzofuran-4-yl)ethanone With dicyclohexylammonium trifloroacetate In 1,4-dioxane at 100℃; for 4h; Stage #2: With sulfuric acid In pentane at 67℃; Inert atmosphere; |
-
-
62803-47-8
6-hydroxy-1-indanone

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium carbonate / acetone / 5 h / Reflux 2.1: N,N-dimethyl-aniline / Inert atmosphere; Reflux 2.2: 25 °C 3.1: ozone / dichloromethane / -78 - 20 °C / Inert atmosphere 3.2: 20 °C 4.1: toluene-4-sulfonic acid / toluene / 0.75 h / Reflux 5.1: hydrogen / triethylamine; 5% Pd(II)/C(eggshell) / ethanol; tetrahydrofuran / 24 h / 35 °C / 4500.45 - 5250.53 Torr View Scheme |

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: N,N-dimethyl-aniline / Inert atmosphere; Reflux 1.2: 25 °C 2.1: ozone / dichloromethane / -78 - 20 °C / Inert atmosphere 2.2: 20 °C 3.1: toluene-4-sulfonic acid / toluene / 0.75 h / Reflux 4.1: hydrogen / triethylamine; 5% Pd(II)/C(eggshell) / ethanol; tetrahydrofuran / 24 h / 35 °C / 4500.45 - 5250.53 Torr View Scheme |

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: ozone / dichloromethane / -78 - 20 °C / Inert atmosphere 1.2: 20 °C 2.1: toluene-4-sulfonic acid / toluene / 0.75 h / Reflux 3.1: hydrogen / triethylamine; 5% Pd(II)/C(eggshell) / ethanol; tetrahydrofuran / 24 h / 35 °C / 4500.45 - 5250.53 Torr View Scheme |
-
-
1290145-09-3
2-methoxy-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-one

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: toluene-4-sulfonic acid / toluene / 0.75 h / Reflux 2: hydrogen / triethylamine; 5% Pd(II)/C(eggshell) / ethanol; tetrahydrofuran / 24 h / 35 °C / 4500.45 - 5250.53 Torr View Scheme |
-
-
198707-57-2
3-(2,3-dihydro-1-benzofuran-5-yl)prop-2-enoic acid

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: thionyl chloride / 0.67 h / 26 - 73 °C / Heating / reflux 2.1: hydrogen / nickel / methanol / 4 h / 50 °C 3.1: bromine; sodium acetate; acetic acid / 14 h / 20 - 28 °C 4.1: thionyl chloride / N,N-dimethyl-formamide / 1,2-dichloro-benzene / 2 h / 28 - 60 °C 4.2: 1 h / -5 - 5 °C 5.1: hydrogen; sodium acetate / raney chloride / methanol / 50 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: sodium hydroxide; hydrogen; ammonium formate / palladium 10% on activated carbon / water / 5 - 6 h / 20 °C 2.1: bromine; sodium acetate; acetic acid / 2 - 3 h / 0 - 45 °C 3.1: thionyl chloride / N,N-dimethyl-formamide / 1 - 2 h / 20 °C 3.2: 1 - 2 h / 0 - 5 °C 4.1: hydrogen; sodium acetate; acetic acid / palladium 10% on activated carbon / 1471.14 - 2206.72 Torr View Scheme |
-
-
217483-05-1
ethyl 3-(2,3-dihydrobenzofuran-5-yl)acrylate

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: hydrogen / nickel / methanol / 4 h / 50 °C 2.1: bromine; sodium acetate; acetic acid / 14 h / 20 - 28 °C 3.1: thionyl chloride / N,N-dimethyl-formamide / 1,2-dichloro-benzene / 2 h / 28 - 60 °C 3.2: 1 h / -5 - 5 °C 4.1: hydrogen; sodium acetate / raney chloride / methanol / 50 °C View Scheme |
-
-
215057-28-6
3-(2,3-dihydro-1-benzofuran-5-yl)propanoic acid

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: bromine; sodium acetate; acetic acid / 2 - 3 h / 0 - 45 °C 2.1: thionyl chloride / N,N-dimethyl-formamide / 1 - 2 h / 20 °C 2.2: 1 - 2 h / 0 - 5 °C 3.1: hydrogen; sodium acetate; acetic acid / palladium 10% on activated carbon / 1471.14 - 2206.72 Torr View Scheme |
-
-
209256-42-8
2,3-dihydro-1-benzofuran-4-carbaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: tetrahydrofuran / 6.5 h / -10 - 20 °C 2: Dess-Martin periodane / dichloromethane / 2 h / 20 °C 3: copper(II) bis(trifluoromethanesulfonate) / 1,2-dichloro-ethane / 16 h / 10 - 20 °C View Scheme |
-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

-
-
1092507-07-7
2,6,7,8-tetrahydro-1H-indeno[5,4-b]furan-8-ol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran; methanol at 0 - 20℃; for 6h; | 100% |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.366667h; Green chemistry; | 98% |
-
-
387-45-1
2-chloro-6-fluorobenzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.166667h; Green chemistry; | 98% |
| With sodium hydroxide In water |
-
-
89-98-5
2-chloro-benzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.333333h; Green chemistry; | 97% |
| With sodium hydroxide In water |
-
-
6334-18-5
2,3-dichlorobenzylaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.483333h; Green chemistry; | 96% |
-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.6h; Green chemistry; | 96% |

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In 1,2-dimethoxyethane; ethanol at 0 - 60℃; for 18h; | 95% |
-
-
66-77-3
1-naphthaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.516667h; Green chemistry; | 95% |
-
-
874-42-0
2,4-dichlorobenzaldeyhde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.583333h; Green chemistry; | 95% |
-
-
100-52-7
benzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.5h; Reagent/catalyst; Green chemistry; | 94% |
| With sodium hydroxide In water |
-
-
83-38-5
2,6-dichlorobenzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.583333h; Green chemistry; | 94% |
| With sodium hydroxide In water |
-
-
372-09-8
cyanoacetic acid

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

-
-
221530-44-5
2-(1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile

| Conditions | Yield |
|---|---|
| With piperidine at 100 - 110℃; | 93% |
-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.666667h; Green chemistry; | 93% |
-
-
122-03-2
(4-isopropylbenzaldehyde)

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.5h; Green chemistry; | 91% |
-
-
1489-69-6
Cyclopropanecarboxaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.416667h; Green chemistry; | 90% |

| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid In toluene Reagent/catalyst; Solvent; | 89.8% |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

-
-
221530-44-5
2-(1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ylidene)acetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran; mineral oil at 20℃; for 0.5h; Horner-Wadsworth-Emmons olefination; Stage #2: 1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one In tetrahydrofuran; mineral oil at 40℃; for 1h; Horner-Wadsworth-Emmons olefination; | 89% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium methylate In tetrahydrofuran at 20℃; for 1h; Stage #2: 1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one In tetrahydrofuran at 20℃; for 2.25h; | 80% |
| Stage #1: diethyl 1-cyanomethylphosphonate With sodium hydride In tetrahydrofuran at 25℃; for 1h; Large scale; Stage #2: 1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one In tetrahydrofuran; water for 3.5h; Large scale; | 5.1 kg |
| In methanol at 20℃; for 0.25h; Alkaline conditions; | |
| With sodium methylate at 5 - 25℃; |
-
-
2537-48-6
diethyl 1-cyanomethylphosphonate

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

-
-
196597-79-2
(E)-(1,6,7,8-tetrahydro-2H-indeno[5,4-b]furan-8-ylidene)acetonitrile

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol; water; toluene | 88% |
| With sodium methylate In methanol; toluene at 20℃; Wittig Reaction; | 88% |
| With sodium methylate In methanol; toluene at 0℃; for 5h; | 84.4% |
-
-
659-28-9
p-trifluoromethoxybenzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.333333h; Green chemistry; | 86% |
-
-
24964-64-5
3-Cyanobenzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.666667h; Green chemistry; | 85% |
-
-
455-19-6
4-Trifluoromethylbenzaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.383333h; Green chemistry; | 83% |
-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

-
-
1093972-05-4
ethyl (2)-1,2,6,7-tetrahydro-8H-indeno[5,4-b]furan-8-ylideneacetate

| Conditions | Yield |
|---|---|
| Stage #1: diethoxyphosphoryl-acetic acid ethyl ester With sodium hydride In toluene at 0 - 5℃; for 2h; Stage #2: 1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one In toluene at 90 - 100℃; for 15 - 18h; | 80% |
| Stage #1: diethoxyphosphoryl-acetic acid ethyl ester With sodium hydride In toluene; mineral oil at 0 - 20℃; Inert atmosphere; Stage #2: 1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one In toluene; mineral oil at 90 - 100℃; Inert atmosphere; |
-
-
115262-01-6
[bromo(difluoro)methyl](trimethyl)silane

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Stage #1: [bromo(difluoro)methyl]-trimethyl-silane; 1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one With tetrabutylammomium bromide In toluene at 110℃; for 7h; Sealed tube; Stage #2: With tetrabutyl ammonium fluoride In toluene at 20℃; for 2h; | 80% |
-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

-
-
456-48-4
3-Fluorobenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.433333h; Green chemistry; | 80% |
-
-
73568-25-9
2-chloro-3-quinoline carboxaldehyde

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 0.3h; Green chemistry; | 75% |
-
-
867-13-0
diethoxyphosphoryl-acetic acid ethyl ester

-
-
196597-78-1
1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one

| Conditions | Yield |
|---|---|
| Stage #1: diethoxyphosphoryl-acetic acid ethyl ester With sodium hydride In toluene at 20℃; for 2h; Cooling with ice; Stage #2: 1,2,6,7-tetrahydro-8H-indeno[5,4-b]-furan-8-one In toluene at 90℃; Cooling with ice; | 74% |
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one Chemical Properties
The Molecular Structure of 1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one (CAS NO.196597-78-1):
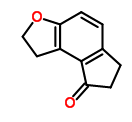
Empirical Formula: C11H10O2
Molecular Weight: 174.1959
Nominal Mass: 174 Da
Average Mass: 174.1959 Da
Monoisotopic Mass: 174.06808 Da
Index of Refraction: 1.624
Molar Refractivity: 47.726 cm3
Molar Volume: 135.156 cm3
Surface Tension: 53.251 dyne/cm
Density: 1.289 g/cm3
Flash Point: 165.595 °C
Enthalpy of Vaporization: 57.709 kJ/mol
Boiling Point: 334.156 °C at 760 mmHg
Vapour Pressure: 0 mmHg at 25°C
Product Categories: Aromatics Compounds;Aromatics;Heterocycles
Appearance: White Solid
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one Uses
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one (CAS NO.196597-78-1) can be used as receptor agonist and a therapeutic agent for sleep disorders.
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one Specification
1,2,6,7-Tetrahydro-8H-indeno[5,4-b]furan-8-one (CAS NO.196597-78-1) is also called as 1,2,6,7-Tetrahydro-8H-indeno[5,4-β]furan-8-one .
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 196597-81-6
- 196597-86-1
- 19660-16-3
- 196601-68-0
- 196601-69-1
- 19660-77-6
- 196612-93-8
- 196613-57-7
- 196618-13-0
- 19662-59-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View