-
Name
1-Pentanethiol
- EINECS 203-789-1
- CAS No. 110-66-7
- Article Data25
- CAS DataBase
- Density 0.832 g/cm3
- Solubility practically insoluble in water
- Melting Point -76 °C
- Formula C5H12S
- Boiling Point 126.3 °C at 760 mmHg
- Molecular Weight 104.216
- Flash Point 18.3 °C
- Transport Information UN 1111 3/PG 2
- Appearance clear colorless to light-yellow liquid
- Safety 16-23-24/25-37/39-26
- Risk Codes 11-20/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xn
Xn
- Synonyms Amylthioalcohol;Mercaptopentane;Pentyl mercaptan;Pentylthiol;n-Amyl mercaptan;n-Amylthiol;n-Pentanethiol;n-Pentyl mercaptan;n-Pentylthiol;Amylmercaptan (7CI);Pentanethiol (6CI);1-Mercaptopentane;1-Pentyl mercaptan;1-n-Pentanethiol;Amyl hydrosulfide;Amyl sulfhydrate;
- PSA 38.80000
- LogP 2.10640
Synthetic route
-
-
32446-40-5
1-thiocyanatopentane

-
-
110-66-7
n-pentanethiol

| Conditions | Yield |
|---|---|
| With tetraphosphorus decasulfide In toluene for 1.5h; Reflux; | 87% |

-
-
110-66-7
n-pentanethiol

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; zinc In dichloromethane at 25℃; for 0.166667h; | 85% |

-
-
110-66-7
n-pentanethiol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol at 20℃; for 1h; | 70% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium iodide; 1-(2-hydroxyethyl)-4,6-diphenylpyridin-2-thione In acetonitrile for 26h; Ambient temperature; | 61% |

| Conditions | Yield |
|---|---|
| With phosphorous (V) sulfide; benzene Erhitzen des Reaktionsprodukts auf 190grad; |

| Conditions | Yield |
|---|---|
| With sodium hydrogensulfide; ethanol | |
| With ethanol; potassium hydrosulfide | |
| With potassium hydrosulfide |

| Conditions | Yield |
|---|---|
| With water Zersetzen des Thiuroniumsalzes mit starker KOH auf dem Wasserbad; |
-
-
56342-26-8
cyano-dithiocarbonimidic acid dipentyl ester

-
-
110-66-7
n-pentanethiol

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
110-89-4
piperidine

-
A
-
1613-51-0
sulfure de pentamethylene

-
B
-
1795-09-1
2-methylthiolane

-
C
-
110-86-1
pyridine

-
D
-
10324-58-0
1-pentylpiperidine

-
E
-
110-66-7
n-pentanethiol

| Conditions | Yield |
|---|---|
| With hydrogen sulfide; hydrogen; sulfidized Ni-W at 300℃; under 105008 Torr; for 5h; Product distribution; | A n/a B n/a C 0.59 % Chromat. D 3.59 % Chromat. E n/a |

-
-
22537-07-1
4-pentenyl-1-amine

-
A
-
1613-51-0
sulfure de pentamethylene

-
B
-
1795-09-1
2-methylthiolane

-
C
-
872-10-6
diamyl sulfide

-
D
-
110-66-7
n-pentanethiol

-
E
-
17651-37-5
pent-4-ene-1-thiol

| Conditions | Yield |
|---|---|
| With hydrogen sulfide; hydrogen; sulfidized Ni-W at 300℃; for 5h; Product distribution; |

-
-
35452-30-3
1-pentanesulfonic acid

-
-
110-66-7
n-pentanethiol

| Conditions | Yield |
|---|---|
| With diphosphorus pentasulfide; water 1.) sulfolane, 90 deg C for 24 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With diphosphorus pentasulfide; lithium aluminium tetrahydride 1.) sulfolane, 90 deg C for 24 h 2.) ether, room temperature for 30 min; Yield given. Multistep reaction; |
-
-
110-58-7
1-pentanamine

-
A
-
872-10-6
diamyl sulfide

-
B
-
110-66-7
n-pentanethiol

-
C
-
2050-92-2
Di-n-amylamine

-
D
-
109-66-0
pentane

| Conditions | Yield |
|---|---|
| With hydrogen sulfide; hydrogen; sulfidized Ni-W at 300℃; for 5h; Product distribution; | A n/a B n/a C 2.3 % Chromat. D 3.2 % Chromat. |

-
-
7647-01-0
hydrogenchloride

-
A
-
110-66-7
n-pentanethiol

-
B
-
855395-51-6
S,S-dipentyl 1,2-ethanebisthioate

-
D
-
144-62-7
oxalic acid

-
-
22537-07-1
4-pentenyl-1-amine

-
A
-
1613-51-0
sulfure de pentamethylene

-
B
-
1795-09-1
2-methylthiolane

-
C
-
78-78-4
methylbutane

-
D
-
110-66-7
n-pentanethiol

-
E
-
563-46-2
2-Methyl-1-butene

-
F
-
109-66-0
pentane

| Conditions | Yield |
|---|---|
| With hydrogen sulfide; hydrogen Product distribution; |


| Conditions | Yield |
|---|---|
| With hydrogen sulfide; TK-554 catalyst at 120 - 220℃; Product distribution / selectivity; |

-
-
554-14-3
2-Methylthiophene

-
A
-
1795-09-1
2-methylthiolane

-
B
-
110-66-7
n-pentanethiol

-
C
-
109-67-1
1-penten

| Conditions | Yield |
|---|---|
| With nonane; hydrogen |

-
-
554-14-3
2-Methylthiophene

-
A
-
1795-09-1
2-methylthiolane

-
B
-
110-66-7
n-pentanethiol

-
C
-
109-67-1
1-penten

-
D
-
109-66-0
pentane

| Conditions | Yield |
|---|---|
| With nonane; hydrogen at 224.84℃; |


| Conditions | Yield |
|---|---|
| With nonane; platinum on alumina; hydrogen at 224.84℃; |

| Conditions | Yield |
|---|---|
| In ethanol; water the thiol was added to soln. of Hg(NO3)2 in EtOH/H2O in a Schlenk tube; soln. was stirred for 12 h in the dark; ppt. was filtered off, washed with cold EtOH and dried in vacuo; | 100% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; sodium iodide In acetonitrile at 25℃; | 100% |
| With tert.-butylhydroperoxide; sodium iodide In acetonitrile at 20℃; for 12h; | 99% |
| With ammonium iodide In acetonitrile at 20℃; for 3h; Electrochemical reaction; | 78% |

| Conditions | Yield |
|---|---|
| With water; dihydrogen peroxide In various solvent(s) at 20℃; for 0.166667h; Oxidation; Dimerization; | 99% |
| With aluminum oxide In neat (no solvent) for 0.333333h; Milling; chemoselective reaction; | 98% |
| With oxygen; SiO2-Cl In dichloromethane at 0℃; for 0.166667h; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: n-pentanethiol With sodium hydroxide In dichloromethane for 1h; Stage #2: propargyl bromide In dichloromethane; toluene for 2h; | 99% |
| With potassium hydroxide |
-
-
110-66-7
n-pentanethiol

-
-
115975-33-2
N,N-Dimethyl-2,4-bis(trifluoroacetyl)-1-naphthylamine

-
-
127053-12-7
2,2,2-Trifluoro-1-[4-pentylsulfanyl-3-(2,2,2-trifluoro-acetyl)-naphthalen-1-yl]-ethanone

| Conditions | Yield |
|---|---|
| In acetonitrile for 41h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With 9-borabicyclo[3.3.1]nonane dimer In tetrahydrofuran at 20℃; for 3h; | 99% |
-
-
110-66-7
n-pentanethiol

-
-
140645-28-9
(4S)-N-tert-butoxycarbonyl-4-[[(4'-methylbenzenesulfonyl)oxy]methyl]-2,2-dimethyl-1,3-oxazolidine

-
-
935547-63-0
4(S)-2,2-dimethyl-4-(pentylthiomethyl)oxazolidine-3-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: n-pentanethiol With sodium hydride In N,N-dimethyl-formamide at 20℃; Stage #2: (4S)-N-tert-butoxycarbonyl-4-[[(4'-methylbenzenesulfonyl)oxy]methyl]-2,2-dimethyl-1,3-oxazolidine In N,N-dimethyl-formamide at 40℃; for 1h; | 99% |
| With sodium hydride In N,N-dimethyl-formamide at 60 - 80℃; |

| Conditions | Yield |
|---|---|
| With C47H57N4O4PS In diethyl ether at 20℃; for 24h; Michael Addition; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: n-pentanethiol In acetonitrile at 20℃; for 0.0833333h; Stage #2: tert-Butyl acrylate In acetonitrile at 50 - 60℃; for 0.25h; | 99% |
| With potassium carbonate In dichloromethane at 20℃; for 20h; Michael Addition; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With C39H60N4O4; yttrium(III) trifluoromethanesulfonate In dichloromethane at -60℃; for 4h; enantioselective reaction; | 99% |
-
-
110-66-7
n-pentanethiol

-
-
122009-56-7
2-(4-allyl-1-piperazinyl)-4-chloroquinazoline

-
-
134249-89-1
2-(4-Allyl-piperazin-1-yl)-4-pentylsulfanyl-quinazoline

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0℃; | 98% |
-
-
110-66-7
n-pentanethiol

-
-
163080-54-4
12-Chloro-8,9,10,11-tetrahydro-7H-benzo[4,5]imidazo[1,2-a]cyclohepta[d]pyridine-6-carbonitrile

| Conditions | Yield |
|---|---|
| With 5-bromo-2'-deoxyuridine In benzene for 4h; Ambient temperature; | 98% |
-
-
110-66-7
n-pentanethiol

-
-
191540-37-1
prop-2-en-1-yl 2,3,4-tri-O-acetyl-α-L-fucopyranoside

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In 1,4-dioxane at 75℃; | 98% |
-
-
110-66-7
n-pentanethiol

-
-
51-80-9
bis-(dimethylamino)methane

-
-
1369272-96-7
N,N-dimethyl-1-(pentylsulfanyl)methaneamine

| Conditions | Yield |
|---|---|
| With samarium(III) chloride hexahydrate at 60℃; for 1.5h; Inert atmosphere; chemoselective reaction; | 98% |
-
-
5625-90-1
4-(morpholinomethyl)morpholine

-
-
110-66-7
n-pentanethiol

-
-
1394913-18-8
N-(pentylsulfanylmethyl)morpholine

| Conditions | Yield |
|---|---|
| With zinc(II) chloride dihydrate at 60℃; for 6h; Temperature; Reagent/catalyst; Time; | 98% |

| Conditions | Yield |
|---|---|
| With Phenazin; 1,8-diazabicyclo[5.4.0]undec-7-ene In N,N-dimethyl-formamide at 90℃; for 12h; Temperature; Schlenk technique; | 98% |

| Conditions | Yield |
|---|---|
| With C49H53N4O4PS In diethyl ether at 0℃; enantioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: n-pentanethiol; C19H21NO5 In dichloromethane for 0.5h; Stage #2: With Dimethyl(phenyl)phosphine In dichloromethane at 20℃; for 3h; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: thiophene; n-pentanethiol; dimethyl amine With sodium nitrate at 24℃; for 2.83333h; Stage #2: With lead(IV) tetraacetate; nickel dibromide at 18℃; for 4h; Temperature; | 97.8% |


| Conditions | Yield |
|---|---|
| With H-beta(19) zeolite In neat (no solvent) at 23℃; for 6h; | 97% |
| With graphene oxide In neat (no solvent) at 20℃; for 3h; Green chemistry; chemoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With [RuCl(η5-(3-phenyl)indenyl)(PPh3)2] In toluene at 80℃; for 16h; Glovebox; | 96% |
| (i) NiCl2, (ii) /BRN= 1730979/; Multistep reaction; |
-
-
110-66-7
n-pentanethiol

-
-
39213-06-4
4-Chloro-2-(4-methyl-1-piperazinyl)quinazoline

-
-
129663-85-0
2-(4-Methyl-piperazin-1-yl)-4-pentylsulfanyl-quinazoline

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide | 96% |
-
-
110-66-7
n-pentanethiol

-
-
76008-87-2
1-pentanesulfinic acid

| Conditions | Yield |
|---|---|
| With 3,3-dimethyldioxirane In dichloromethane at -40℃; | 96% |

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)(pentamethylcyclopentadiene)ruthenium(II) In acetonitrile at 20℃; for 1h; Alkylation; | 96% |
-
-
110-66-7
n-pentanethiol

-
-
15442-91-8
1,2,4,5-tetrabromomethylbenzene

-
-
261912-38-3
1,2,4,5-tetrakis(pentylsulfanylmethyl)benzene

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 25℃; for 12h; Substitution; | 96% |
-
-
33301-41-6
1-benzhydryl-3-azetidinyl methanesulfonate

-
-
110-66-7
n-pentanethiol

-
-
1309207-50-8
1-(diphenylmethyl)-3-(pentylthio)azetidine

| Conditions | Yield |
|---|---|
| Stage #1: n-pentanethiol With sodium hydride In dimethyl sulfoxide at 0℃; for 0.5h; Inert atmosphere; Stage #2: 1-benzhydryl-3-azetidinyl methanesulfonate In dimethyl sulfoxide at 0 - 20℃; | 96% |

| Conditions | Yield |
|---|---|
| With (N,N′-bis(2,4,6-trimethylphenyl)imidazole-2-ylidene)Pd(acac)Cl; 3-isopropyl-6-methylcyclohexa-1,4-diene; triethylamine In neat (no solvent) at 100℃; for 8h; Green chemistry; regioselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| With (N,N′-bis(2,4,6-trimethylphenyl)imidazole-2-ylidene)Pd(acac)Cl; 3-isopropyl-6-methylcyclohexa-1,4-diene; triethylamine In neat (no solvent) at 100℃; for 8h; Green chemistry; regioselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| With dimethylbromosulphonium bromide at 20℃; for 0.0666667h; regioselective reaction; | 95% |
| With zinc(II) chloride |
-
-
110-66-7
n-pentanethiol

-
-
420121-38-6
methyl (3-phenylprop-2-yn-1-yl) carbonate

| Conditions | Yield |
|---|---|
| With N-methylcyclohexylamine; chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium (II) at 100℃; for 8h; | 95% |
1-PENTANETHIOL Chemical Properties
MF: C5H12S
MW: 104.21
EINECS: 203-789-1
mp: -76°C
bp: 126 °C(lit.)
density: 0.84 g/mL at 25 °C(lit.)
vapor pressure: 27.4 mm Hg ( 37.7 °C)
refractive index: n20/D 1.446(lit.)
Fp: 65 °F
storage temp: Flammables area
Water Solubility: practically insoluble
Sensitive: Air Sensitive
Merck: 611
BRN: 1730979
The structure of 1-PENTANETHIOL is:
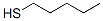
1-PENTANETHIOL Toxicity Data With Reference
1、RTECS#: CAS# 110-66-7: SA3150000
2、LD50/LC50: RTECS: Not available.
3、Carcinogenicity: 1-Pentanethiol - Not listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65.
4、Other: The toxicological properties have not been fully investigated. See actual entry in RTECS for complete information.
|
1-PENTANETHIOL Consensus Reports
1-PENTANETHIOL Safety Profile
Hazard Codes: F,Xn
Risk Statements: 11-20/22-36/37/38
Safety Statements: 16-23-24/25-37/39-26
RIDADR: UN 1111 3/PG 2
WGK Germany: 3
RTECS: SA3150000
F: 9-13-23
HazardClass: 3
PackingGroup: II
Hazardous Substances Data: 110-66-7(Hazardous Substances Data)
Moderately toxic by inhalation. A weak sensitizer and allergen. Local contact may cause contact dermatitis. A flammable liquid and dangerous fire hazard when exposed to heat or flame; can react vigorously with oxidizing materials. Hypergolic reaction with concentrated nitric acid. To fight fire, use foam, CO2, dry chemical.
1-PENTANETHIOL Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid, Poison (UN 1228); DOT Class: 6.1; Label: Poison, Flammable Liquid (UN 3071)
1-PENTANETHIOL Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors can travel to a source of ignition and flash back. Will burn if involved in a fire. Flammable liquid and vapor. Containers may explode if exposed to fire.
Extinguishing Media: Use water spray to cool fire-exposed containers. Use foam, dry chemical, or carbon dioxide.
2、Handling and Storage of 1-PENTANETHIOL
Handling: Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. This product may be under pressure; cool before opening. Storage: Keep away from sources of ignition. Store in a cool, dry place. Keep container closed when not in use. Flammables-area. Store under nitrogen.
Related Products
- 1-PENTANETHIOL
- 1106685-58-8
- 1106685-61-3
- 110668-69-4
- 110674-37-8
- 110-67-8
- 11067-81-5
- 110683-22-2
- 110683-23-3
- 11068-82-9
- 110-68-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View