-
Name
2-(2-Hydroxyethyl)pyridine
- EINECS 203-140-2
- CAS No. 103-74-2
- Article Data38
- CAS DataBase
- Density 1.093 g/cm3
- Solubility soluble in water
- Melting Point -8 - -7°C
- Formula C7H9NO
- Boiling Point 240.6 °C at 760 mmHg
- Molecular Weight 123.155
- Flash Point 92.8 °C
- Transport Information
- Appearance clear yellow to brown liquid
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-(2-Hydroxyethyl)pyridine;2-(2-Pyridyl)ethanol;2-(beta-Hydroxyethyl)pyridine;AI3-52671;HSDB 5357;Pyridine, 2-(2-hydroxyethyl)-;Pyridine-2-ethanol;
- PSA 33.12000
- LogP 0.61640
Synthetic route

-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| With potassium hydrogen difluoride; dihydrogen peroxide In tetrahydrofuran; methanol at 50℃; for 24h; Fleming-Tamao Oxidation; | 80% |

| Conditions | Yield |
|---|---|
| Stage #1: α-picoline With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 1h; Stage #2: 1-Hydroxymethyl-1H-benzotriazole In tetrahydrofuran at -78℃; for 2h; | 65% |
-
-
64364-85-8
2-(hydroxyethyl)pyridine-N-oxide

-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| With tetraethylammonium hexafluorophosphate In water; acetonitrile at 80℃; Inert atmosphere; Electrolysis; | 63% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloromethylpyridine With chloro-trimethyl-silane; ethylene dibromide; zinc In tetrahydrofuran at 70℃; for 2h; Schlenk technique; Inert atmosphere; Stage #2: formaldehyd In tetrahydrofuran at 70℃; for 6h; Schlenk technique; Inert atmosphere; | 59% |
-
-
109-06-8
α-picoline

-
-
50-00-0
formaldehyd

-
A
-
100-69-6
2-vinylpyridine

-
B
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| With sulfuric acid; water; hydrogen at 160℃; |


| Conditions | Yield |
|---|---|
| With water at 120 - 135℃; | |
| With water at 120℃; im geschlossenem Gefaess; | |
| With water at 150℃; im geschlossenem Gefaess; |
-
-
109-06-8
α-picoline

-
-
50-00-0
formaldehyd

-
A
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
B
-
49745-42-8
2-(pyridin-2-yl)propane-1,3-diol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; water at 130 - 136℃; under 7355.08 Torr; |


| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; diethyl ether |

-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With phenyllithium anfangs unter Kuehlung, zuletzt bei Siedetemperatur; |

| Conditions | Yield |
|---|---|
| In 2-methyl-propan-1-ol; isopropyl alcohol |


-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| With potassium fluoride; tetrabutyl ammonium fluoride; dihydrogen peroxide; potassium hydrogencarbonate In tetrahydrofuran; water at 40℃; for 2h; Fleming-Tamao Oxidation; |

-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| With potassium fluoride; tetrabutyl ammonium fluoride; dihydrogen peroxide; potassium hydrogencarbonate In tetrahydrofuran; water at 40℃; for 2h; Fleming-Tamao Oxidation; |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
124-63-0
methanesulfonyl chloride

-
-
138428-37-2
2-(pyridin-2-yl)ethyl methanesulfonate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 3h; | 100% |
| With triethylamine In acetonitrile at 40℃; under 2585.81 Torr; for 0.333333h; Flow reactor; | 100% |
| With triethylamine In dichloromethane at 25℃; for 3.25h; Cooling with ice; | 98% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
108-24-7
acetic anhydride

-
-
16632-09-0
2-methylamino-6-(2-hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In tetrahydrofuran Inert atmosphere; Glovebox; | 100% |
| With dmap; N-ethyl-N,N-diisopropylamine at 20℃; for 0.516667h; Inert atmosphere; Glovebox; | 822 mg |

| Conditions | Yield |
|---|---|
| With N,N'-dimethylaminopyridine; di-tert-butyl dicarbonate In nitromethane at 50℃; for 16h; | 99% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| Stage #1: nickel(II) chloride dihydrate In 1,2-dimethoxyethane for 1.5h; Reflux; Inert atmosphere; Schlenk technique; Stage #2: In dichloromethane Inert atmosphere; Schlenk technique; Stage #3: 2-(2-Hydroxyethyl)pyridine In dichloromethane at 20℃; for 1h; Inert atmosphere; Schlenk technique; | 96% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane | 96% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; for 12h; | 79% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 25℃; for 16h; | 69% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
161227-19-6
2-<(2-tert-butyldimethylsilyloxy)ethyl>pyridine

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 24h; Ambient temperature; | 96% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
72996-65-7
2-(2-Bromoethyl)pyridine hydrobromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide | 95% |
| With hydrogen bromide In acetic acid at 78℃; for 48h; | 73% |
| With hydrogen bromide In water; toluene at 120℃; | 66% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
161227-19-6
2-<(2-tert-butyldimethylsilyloxy)ethyl>pyridine

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 25℃; for 24h; | 95% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
859439-31-9
trimethylindium diethyl ether adduct

| Conditions | Yield |
|---|---|
| In benzene byproducts: CH4, Et2O; under N2; by the react. of In-contg. compd. with a ligand in 1:1 stoich.ratio in benzene; stirring for 4 h at room temp.; the solvent was removed under reduced pressure; recrystn. from benzene/hexane; elem. anal.; | 95% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| In benzene byproducts: C2H6, Et2O; under N2; by the react. of In-contg. compd. with a ligand in 1:1 stoich.ratio in benzene; stirring for 4 h at room temp.; the solvent was removed under reduced pressure; recrystn. from benzene/hexane; elem. anal.; | 95% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| With boric acid In methanol; water | 95% |

| Conditions | Yield |
|---|---|
| In methanol; water to soln. H3BO3 in aq. MeOH (1:1) amine was added, stirred for 10 min, solvent was removed in vacuo, residue was heated in oven to dry 4 h at 100°C; elem. anal.; | 95% |

| Conditions | Yield |
|---|---|
| With (R)-(-)-1-[(S)-2-(dicyclohexylphosphino)ferrocenyl]ethyl di-t-butylphosphine; bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; caesium carbonate In toluene at 90℃; for 16h; Glovebox; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20℃; for 2h; | 94.3% |

| Conditions | Yield |
|---|---|
| Stage #1: naphthalen-1-yl-acetyl chloride; 2-(2-Hydroxyethyl)pyridine In ethyl acetate; toluene at 20℃; for 2h; Stage #2: With sodium carbonate In dichloromethane; water at 20℃; | 94.2% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
13734-41-3
t-Boc-L-valine

-
-
91841-65-5
(S)-2-tert-Butoxycarbonylamino-3-methyl-butyric acid 2-pyridin-2-yl-ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane 1) -20 degC, 10 min.; 2) 20 degC, 3 h; | 94% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
31121-29-6
triethylgallium diethyl ether adduct

| Conditions | Yield |
|---|---|
| In benzene byproducts: C2H6, Et2O; under N2; a soln. of a ligand in benzene was added to a benzene soln. ofGa-contg. compd. and stirred for 24 h; the solvent was removed under reduced pressure; elem. anal.; | 94% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
112704-79-7
2-fluoro-4-bromobenzoic acid

-
-
920270-28-6
2-(2-pyridyl)ethyl 4-bromo-2-fluorobenzoate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; Product distribution / selectivity; | 93% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 16h; Inert atmosphere; | 93% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; Product distribution / selectivity; | 80% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
31121-29-6
triethylgallium diethyl ether adduct

| Conditions | Yield |
|---|---|
| In benzene byproducts: C2H6, Et2O; under N2; a soln. of a ligand in benzene was added to a benzene soln. ofGa-contg. compd. and stirred for 24 h; the solvent was removed under reduced pressure; elem. anal.; | 93% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: CH4, Et2O; under N2; a soln. of a ligand in benzene was added to a benzene soln. ofGa-contg. compd. and stirred for 24 h; the solvent was removed under reduced pressure; elem. anal.; | 93% |

| Conditions | Yield |
|---|---|
| at 90℃; for 0.0833333h; Neat (no solvent); Grinding; | 93% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethyl acetate at 20℃; for 2h; Inert atmosphere; | 92.9% |

| Conditions | Yield |
|---|---|
| With acetic acid In ethyl acetate at 20℃; for 2h; | 92.1% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
2018-66-8
Z-Leu-OH

-
-
91841-60-0
(S)-2-Benzyloxycarbonylamino-4-methyl-pentanoic acid 2-pyridin-2-yl-ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane 1) -20 degC, 10 min.; 2) 20 degC, 3 h; | 92% |

| Conditions | Yield |
|---|---|
| In benzene byproducts: CH4, Et2O; under N2; a soln. of a ligand (5.03 mmol) in benzene was added dropwise to a benzene soln. of Ga-contg. compd. (5.03 mmol) and stirred for 24 h; the solvent was removed under reduced pressure; elem. anal.; | 92% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
7697-28-1
4-bromo-3-methylbenzoic acid

-
-
920270-48-0
2-(2-pyridyl)ethyl 4-bromo-3-methylbenzoate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 16h; Inert atmosphere; | 92% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
1456807-88-7
C22H24N2O6S

-
-
1456808-51-7
4-methyl-3-(4-methylpiperazin-1-yl)-2-oxo-5-(2-(pyridin-2-yl)ethoxy)-2H-chromen-7-yl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 20℃; for 0.333333h; Mitsunobu Displacement; | 91% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
15307-79-6
diclofenac sodium

-
-
6046-93-1
copper(II) acetate monohydrate

| Conditions | Yield |
|---|---|
| In methanol at 50℃; | 91% |

-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

| Conditions | Yield |
|---|---|
| In methanol at 24.84℃; for 6h; | 91% |
-
-
103-74-2
2-(2-Hydroxyethyl)pyridine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
107516-54-1
2-(2-tosylethyl)pyridine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 0 - 20℃; for 24h; Inert atmosphere; Schlenk technique; | 91% |
2-(2-Hydroxyethyl)pyridine Specification
The 2-Ethanol pyridine with CAS registry number of 103-74-2 is also known as 2-(beta-Hydroxyethyl)pyridine. The IUPAC name is 2-Pyridin-2-ylethanol. It belongs to product categories of Pyridine; Pyridines, Pyrimidines, Purines and Pteredines; Amino Acid Derivatives. Its EINECS registry number is 203-140-2. In addition, the formula is C7H9NO and the molecular weight is 123.15. This chemical is a clear yellow to brown liquid that should be sealed in ventilate, cool place away from fire and heat. What's more, it can be used in organic synthesis.
Physical properties about 2-Ethanol pyridine are: (1)ACD/LogP: -0.13; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.32; (4)ACD/LogD (pH 7.4): -0.14; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 13.12; (8)ACD/KOC (pH 7.4): 20.03; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 3; (12)Index of Refraction: 1.539; (13)Molar Refractivity: 35.42 cm3; (14)Molar Volume: 112.9 cm3; (15)Surface Tension: 46.3 dyne/cm; (16)Density: 1.09 g/cm3; (17)Flash Point: 92.8 °C; (18)Enthalpy of Vaporization: 50.46 kJ/mol; (19)Boiling Point: 240.6 °C at 760 mmHg; (20)Vapour Pressure: 0.0204 mmHg at 25 °C.
Preparation of 2-Ethanol pyridine: it is prepared by addition reaction of 2-picoline with formaldehyde. The reaction mixture is stirred and heated to 125 °C for 20 hours with reaction pressure of 0.4 MPa when nitrogen has passed into reactor. At last, product is obtained by collecting fraction at 130-145 °C (2.13 kPa).
Uses of 2-Ethanol pyridine: it is used to produce methanesulfonic acid 2-pyridin-2-yl-ethyl ester by reaction with methanesulfonyl chloride. The reaction occurs with reagent NEt3 and solvent CH2Cl2 for 15 minutes. The yield is about 96.3%.
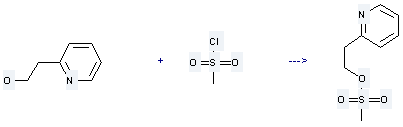
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing. Avoid contact with skin and eyes. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=NC(=C1)CCO
2. InChI: InChI=1S/C7H9NO/c9-6-4-7-3-1-2-5-8-7/h1-3,5,9H,4,6H2
3. InChIKey: BXGYBSJAZFGIPX-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 7750mg/kg (7750mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 57(9-10), Pg. 64, 1992. | |
| rat | LD50 | oral | 9500mg/kg (9500mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 57(9-10), Pg. 64, 1992. |
Related Products
- 2-(2-Hydroxyethyl)pyridine
- 103744-84-9
- 103745-39-7
- 1037479-36-9
- 103750-03-4
- 1037-50-9
- 103-75-3
- 103753-78-2
- 103754-45-6
- 103754-49-0
- 103755-52-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View