-
Name
2',5'-Dihydroxyacetophenone
- EINECS 207-716-4
- CAS No. 490-78-8
- Article Data89
- CAS DataBase
- Density 1.291 g/cm3
- Solubility insoluble in water
- Melting Point 204-206 °C(lit.)
- Formula C8H8O3
- Boiling Point 329.2 °C at 760 mmHg
- Molecular Weight 152.15
- Flash Point 167.1 °C
- Transport Information
- Appearance yellow to yellow-green crystals
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3,5-Dihydroxy acetophenone;2,5-dihydroxyacetophenone;Acetophenone,2',5'-dihydroxy- (7CI,8CI);1-(2,5-Dihydroxyphenyl)ethanone;1-Acetyl-2,5-dihydroxybenzene;2,5-Dihydroxy-1-acetylbenzene;2-Acetyl-1,4-benzenediol;2-Acetylhydroquinone;NSC 3759;Quinacetophenone;
- PSA 57.53000
- LogP 1.30040
Synthetic route

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate for 0.0333333h; microwave irradiation; | 98% |
| With iron(III) chloride for 0.0166667h; Friedel Crafts acylation; Microwave irradiation; regioselective reaction; | 98% |
| With aluminum oxide; methanesulfonic acid at 120℃; for 0.333333h; | 85% |

| Conditions | Yield |
|---|---|
| Stage #1: acetic anhydride; hydroquinone In 1,2-dichloro-ethane at 100℃; for 4h; Friedel-Crafts Acylation; Stage #2: With boron trifluoride diethyl etherate In 1,2-dichloro-ethane at 120℃; for 3h; | 92% |
| zinc(II) chloride at 145 - 150℃; for 0.25h; | 76% |

| Conditions | Yield |
|---|---|
| With ammonium persulfate; silver nitrate In water; acetonitrile at 70℃; for 2h; | 92% |

| Conditions | Yield |
|---|---|
| for 0.116667h; Rearrangement; microwave irradiation; | 90% |
| Stage #1: benzene-1,4-diyl diacetate With aluminum (III) chloride; sodium chloride at 140 - 195℃; for 0.15h; Stage #2: With hydrogenchloride; water for 0.5h; Reflux; | 85% |
| Stage #1: benzene-1,4-diyl diacetate With aluminum (III) chloride; sodium chloride at 180℃; for 3h; Fries rearrangement; Stage #2: With hydrogenchloride In methanol Reflux; | 76.6% |
-
-
40815-75-6
1-(5-allyloxy-2-hydroxyphenyl)-1-ethanone

-
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With bis(benzonitrile)palladium(II) dichloride In benzene for 20h; Heating; | 89% |
-
-
111-43-3
Dipropyl ether

-
-
1205-91-0
benzene-1,4-diyl diacetate

-
A
-
490-78-8
2,5-Dihydroxyacetophenone

-
B
-
288074-68-0
2-hydroxy-5-propoxyacetophenone

| Conditions | Yield |
|---|---|
| With boron trifluoride for 1h; Fries rearrangement; Heating; | A 89% B 9% |


| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate for 0.025h; microwave irradiation; | 85% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 5h; Michael reaction; | A n/a B 80% C 17% |
| In dichloromethane at 20℃; for 5h; Arylation; | A n/a B 51% C 17% |

-
-
1125-55-9
2-Acetyl-1,4-benzoquinone

-
-
55562-82-8
trimethyl(2-methyl-2-propenyl)stannane

-
A
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| In benzene | A 6% B 77% |


| Conditions | Yield |
|---|---|
| With boron dimethyl-trifluoro sulphide In dichloromethane at 20℃; for 1.5h; | 74% |
| With carbon disulfide; aluminum tri-bromide | |
| With pyridine hydrochloride |

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 140℃; for 2h; Fries rearrangement; | 71% |
| Fries rearrangement; |

| Conditions | Yield |
|---|---|
| With zinc at 47 - 48℃; for 0.0166667h; Friedel-Crafts acylation; microwave irradiation; | 70% |
| With aluminium trichloride; nitrobenzene |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In benzene for 3h; crossover Friess rearrangement; Heating; | 65% |
-
-
79950-56-4
5'-cinnamyloxy-2'-hydroxyacetophenone

-
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| at 220℃; for 20h; | 64% |
-
-
1201-38-3
2',5'-dimethoxyacetophenone

-
A
-
705-15-7
1-(2-hydroxy-5-methoxyphenyl)ethan-1-one

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With boron dimethyl-trifluoro sulphide In dichloromethane at 0℃; for 0.166667h; | A 64% B 27% |
-
-
1125-55-9
2-Acetyl-1,4-benzoquinone

-
A
-
68157-88-0
2-Acetyl-3-(3-acetyl-4-hydroxyphenoxy)-1,4-benzoquinone

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With rose bengal In acetonitrile for 8h; Irradiation; Yields of byproduct given; | A 60% B n/a |
-
-
1205-91-0
benzene-1,4-diyl diacetate

-
-
108-95-2
phenol

-
A
-
118-93-4
o-hydroxyacetophenone

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In benzene for 0.5h; cross Fries reaction; microwave irradiation; | A 25% B 60% |

-
-
1125-55-9
2-Acetyl-1,4-benzoquinone

-
A
-
19278-82-1
5-hydroxy-2,3-dihydrobenzofuran-3-one

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| In benzene for 72h; Irradiation; | A 50% B 45% |
| In benzene for 72h; Mechanism; Irradiation; | A 50% B 45% |
-
-
90-15-3
α-naphthol

-
-
1205-91-0
benzene-1,4-diyl diacetate

-
A
-
711-79-5
1-hydroxy-2-acetonaphthone

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In benzene for 0.5h; cross Fries reaction; microwave irradiation; | A 20% B 50% |

-
-
1205-91-0
benzene-1,4-diyl diacetate

-
-
135-19-3
β-naphthol

-
A
-
574-19-6
1-(2-hydroxy-1-naphthyl)ethan-1-one

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In benzene for 0.5h; cross Fries reaction; microwave irradiation; | A 35% B 45% |

-
-
108-73-6
3,5-dihydroxyphenol

-
-
1205-91-0
benzene-1,4-diyl diacetate

-
A
-
480-66-0
2,4,6-trihydroxyacetophenone

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In benzene for 0.5h; cross Fries reaction; microwave irradiation; | A 35% B 40% |

-
-
1125-55-9
2-Acetyl-1,4-benzoquinone

-
-
762-73-2
trimethyl(allyl)stannane

-
A
-
40815-79-0
2-acetyl-3-prop-2'-enyl-1,4-hydroquinone

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| In benzene for 5h; | A 27% B 36% C 23% |
| In benzene dissolved acylquinone and allylstannane stood for 5 h under Ar; concd.; column chromy. (silica gel); eluted (hexane/ether); NMR; IR; mass spectra; elem. anal.; | A 27% B 36% C 23% |

-
-
1205-91-0
benzene-1,4-diyl diacetate

-
A
-
20129-52-6
1,4-diacetyl-2,5-dihydroxybenzene

-
B
-
21222-04-8
5'-Acetoxy-2'-hydroxyacetophenone

-
C
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| In methanol for 12h; Irradiation; | A 10% B 15% C 35% |

-
-
98-86-2
acetophenone

-
A
-
121-71-1
3-Hydroxyacetophenone

-
B
-
85918-30-5
2,3,6-trihydroxyacetophenone

-
C
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; hydrogen fluoride; antimony pentafluoride at -35℃; for 0.5h; Mechanism; Product distribution; | A 31% B 4% C 18% |
| With dihydrogen peroxide; hydrogen fluoride; antimony pentafluoride at -35℃; for 0.5h; | A 31% B 4% C 18% |

-
-
75925-74-5, 94842-18-9
4a,8a-cis-4a-acetyl-4a,5,8,8a-tetrahydro-5,8-methano-1,4-naphthoquinone

-
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With pyridine In xylene Heating; | 29% |

-
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| In pyridine; xylene at 120℃; for 3h; | 29% |
-
-
1125-55-9
2-Acetyl-1,4-benzoquinone

-
-
24850-33-7
allyltributylstanane

-
A
-
40815-79-0
2-acetyl-3-prop-2'-enyl-1,4-hydroquinone

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| In benzene for 1.5h; | A 29% B 10% C 10% |
| In benzene dissolved acylquinone and allylstannane stood for 1.5 h under Ar; concd.; column chromy. (silica gel); eluted (hexane/ether); NMR; IR; mass spectra; elem. anal.; | A 29% B 10% C 10% |


| Conditions | Yield |
|---|---|
| With (Z/E)-2-bromo-2-[4-oxothiazolidin-2-ylidene]-N-phenylacetamide; dimethyl sulfoxide for 3h; Reflux; | 19% |
-
-
1125-55-9
2-Acetyl-1,4-benzoquinone

-
-
762-73-2
trimethyl(allyl)stannane

-
A
-
40815-79-0
2-acetyl-3-prop-2'-enyl-1,4-hydroquinone

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| In acetonitrile dissolved acylquinone and allylstannane stood for 10 min under Ar; concentrated; column chromy. (silica gel); eluted (hexane/ether); NMR; IR; mass spectra; elem. anal.; | A 18% B 18% |

-
-
1205-91-0
benzene-1,4-diyl diacetate

-
A
-
21222-04-8
5'-Acetoxy-2'-hydroxyacetophenone

-
B
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 190 - 200℃; | |
| Stage #1: benzene-1,4-diyl diacetate With aluminium trichloride; sodium chloride at 195℃; for 0.15h; Fries rearrangement; Stage #2: With hydrogenchloride In water for 12h; Further stages. Title compound not separated from byproducts.; | |
| Stage #1: benzene-1,4-diyl diacetate With aluminum (III) chloride; sodium chloride at 140 - 195℃; for 0.15h; Stage #2: With hydrogenchloride; water |
-
-
76200-27-6
ethyl (E)-4-methyl-3,5-hexadienoate

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
72826-95-0, 80866-98-4
(4aR,5R,8aS)-4a-Acetyl-5-(2-benzyloxy-ethyl)-6-methyl-4a,5,8,8a-tetrahydro-[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| With silver(l) oxide Ambient temperature; Absence of light; | 100% |

| Conditions | Yield |
|---|---|
| With silver(l) oxide Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With silver(l) oxide Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With silver(l) oxide for 72h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With silver(l) oxide for 168h; Ambient temperature; | 100% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
3557-22-0
1-(2-hydroxy-5-((tetrahydro-2H-pyran-2-yl)oxy)phenyl)ethan-1-one

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In dichloromethane at 20℃; | 99% |
| With pyridinium p-toluenesulfonate In dichloromethane at 20℃; for 4h; | 91% |
| With pyridinium p-toluenesulfonate In dichloromethane at 20℃; for 5h; | 87% |
-
-
100-39-0
benzyl bromide

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
21766-81-4
1-[2',5'-bis(phenylmethoxy)phenyl]ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 18h; Etherification; Heating; | 99% |
| With potassium carbonate In acetone for 24h; Reflux; | 94% |
| Stage #1: 2,5-Dihydroxyacetophenone In 1,2-dimethoxyethane at 80℃; Inert atmosphere; Stage #2: benzyl bromide In 1,2-dimethoxyethane; N,N-dimethyl-formamide for 12h; | 93% |

| Conditions | Yield |
|---|---|
| With kiwi juice In water at 20℃; for 0.0833333h; Reagent/catalyst; Knoevenagel Condensation; Sonication; Green chemistry; | 99% |
| With lithium perchlorate for 0.0194444h; Irradiation; | 83% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
490-78-8
2,5-Dihydroxyacetophenone

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -30 - -10℃; for 0.666667h; Inert atmosphere; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran 0 deg C to room temp., 0.5 to 1 h; | 98% |

| Conditions | Yield |
|---|---|
| With lithium perchlorate; acetic acid In nitromethane for 16h; electrolysis; | 97% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
843644-58-6
5'-t-butyldimethylsilyloxy-2'-hydroxyacetophenone

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 12h; | 97% |
-
-
118-93-4
o-hydroxyacetophenone

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
849928-22-9
tert-butyl 4-oxo-3,4-dihydro-1'H-spiro[chromene-2,4'-piperidine]-1'-carboxylate

| Conditions | Yield |
|---|---|
| With pyrrolidine In methanol for 24h; | 97% |
-
-
106-95-6
allyl bromide

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
40815-75-6
1-(5-allyloxy-2-hydroxyphenyl)-1-ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 18h; Heating; | 96% |
| With potassium carbonate; sodium iodide In acetone for 15h; Heating; | 86% |
| With potassium carbonate In acetone for 18h; Inert atmosphere; Reflux; | 86% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide In dichloromethane for 0.25h; Ambient temperature; | 95% |
| With oxygen In chloroform; water at 20℃; under 760.051 Torr; for 6h; | 88% |
| With cerium(IV) ammonium nitrate; silica gel In dichloromethane for 0.25h; | 84% |
-
-
100-39-0
benzyl bromide

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
30992-63-3
5-benzyloxy-2-hydroxyacetophenone

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate; potassium iodide In toluene at 115℃; for 14h; | 95% |
| With potassium carbonate In acetone for 48h; Reflux; | 92% |
| With sodium hydrogencarbonate In ethanol at 0 - 10℃; for 3.33333h; | 88.3% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 24h; Heating; | 95% |
-
-
1117-52-8
6,10,14-trimethyl-5,9,13-pentadecatriene-2-on

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
150035-61-3
6-Hydroxy-2-methyl-2-((3E,7E)-4,8,12-trimethyl-trideca-3,7,11-trienyl)-chroman-4-one

| Conditions | Yield |
|---|---|
| With pyrrolidine; 3 A molecular sieve In ethanol Heating; | 95% |
-
-
3282-30-2
pivaloyl chloride

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
309721-51-5
2,2-dimethylpropanoic acid 2-acetyl-1,4-phenylene ester

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 95% |
| With pyridine at 20℃; for 18h; |
-
-
122-51-0
orthoformic acid triethyl ester

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
38445-24-8
6-hydroxy-4-oxo-4H-1-benzopyran

| Conditions | Yield |
|---|---|
| With perchloric acid In water at 20 - 100℃; for 1.08333h; | 95% |
| With perchloric acid at 20℃; for 1.5h; | 78% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In dichloromethane at 0℃; Inert atmosphere; Reflux; | 94.9% |
-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
25015-91-2
2-bromo-1-(2,5-dihydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With aluminium trichloride; Montmorillonite K10; bromine In ethyl acetate at 20℃; for 2h; | 94% |
| With urea-hydrogen peroxide; acetic acid; sodium bromide; silica gel at 120 - 122℃; for 0.00611111h; microwave irradiation; | 71% |
| With copper(I) bromide In chloroform; ethyl acetate for 6h; Reflux; | 6% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
1431642-68-0
1-(2,5-bis(tert-butyldimethylsilyloxy)phenyl)ethanone

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 50℃; for 16h; Inert atmosphere; | 93.2% |
-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
911227-48-0
tert-butyl 6-hydroxy-4-oxospiro[chroman-2,4′-piperidine]-1′-carboxylate

| Conditions | Yield |
|---|---|
| With pyrrolidine In methanol at 60℃; for 48h; | 93% |
| With pyrrolidine In methanol for 18h; Reflux; | 87% |
| With pyrrolidine In methanol for 23h; Reflux; | 82% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
69367-11-9
6-Hydroxy-2-methyl-2-(4-methyl-3-pentenyl)-4-chromanon

| Conditions | Yield |
|---|---|
| With pyrrolidine; 3 A molecular sieve In ethanol Heating; | 92% |
-
-
490-78-8
2,5-Dihydroxyacetophenone

-
-
100-46-9
benzylamine

-
-
82488-61-7
2,5-Dihydroxyphenylethylidenebenzylamine

| Conditions | Yield |
|---|---|
| In toluene Heating; or ZnCl2, 1) 30 min, 160 deg C, 2) 5 min, 180 deg C; | 92% |
| In toluene Heating; | 90% |

| Conditions | Yield |
|---|---|
| With piperidine for 0.166667h; Neat (no solvent); Microwave irradiation; | 92% |
| With pyrrolidine In ethanol Reflux; | 92% |
| With Amberlite IRA 400 basic resin for 0.133333h; Microwave irradiation; Neat (no solvent); | 86% |
| With pyrrolidine In isopropyl alcohol for 2h; Mechanism; Heating; other acetophenones; | 72% |
| With pyrrolidine In isopropyl alcohol for 2h; Heating; | 72% |
2',5'-Dihydroxyacetophenone Chemical Properties
Molar mass:152.14732 g/mol
Structure :
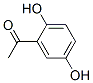
Synonyms of 2',5'-Dihydroxyacetophenone(490-78-8):2-Acetylhydroquinone;Quinacetophenone;2,5-Dihydroxyacetophenone;Acetylquinol;Acetylhydroquinone;Acetophenone, 2',5'-dihydroxy-;Ethanone, 1-(2,5-dihydroxyphenyl)-
Density:1.291 g/cm3
Flash Point:167.1 °C
Boiling Point:329.2 °C at 760 mmHg
Index of Refraction:1.595
Vapour Pressure:9.41E-05 mmHg at 25°C
Melting point:204-206 °C
Water solubility:insoluble
Solubility:dioxane: 50 mg/mL, clear
2',5'-Dihydroxyacetophenone Uses
2',5'-Dihydroxyacetophenone Production
2',5'-Dihydroxyacetophenone Toxicity Data With Reference
| 1. | ipr-mus LDLo:500 mg/kg | CBCCT* “Summary Tables of Biological Tests“ National Research Council Chemical-Biological Coordination Center. 5 (1953),140. |
Other: See actual entry in RTECS for complete information.
2',5'-Dihydroxyacetophenone Consensus Reports
2',5'-Dihydroxyacetophenone Safety Profile
Hazard Codes:Xi

Risk Statements:
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements:
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
2',5'-Dihydroxyacetophenone Specification
Conditions to Avoid:strong oxidants,incompatible materials.
Chemical Stability:Stable under normal temperatures and pressures.
Incompatibilities with Other Materials:strong bases,strong oxidizing agents.
Hazardous Decomposition Products:Irritating and toxic fumes and gases,Carbon monoxide.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 490-79-9
- 490-83-5
- 490-91-5
- 490-97-1
- 4909-78-8
- 4910-06-9
- 4910-40-1
- 4910-47-8
- 491-07-6
- 4911-65-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View