-
Name
2-Bromofluorobenzene
- EINECS 214-018-3
- CAS No. 1072-85-1
- Article Data42
- CAS DataBase
- Density 1.593 g/cm3
- Solubility insoluble in water
- Melting Point -8°C
- Formula C6H4BrF
- Boiling Point 151.5 °C at 760 mmHg
- Molecular Weight 175
- Flash Point 50.2 °C
- Transport Information UN 1993 3/PG 3
- Appearance clear colourless to light yellow liquid
- Safety 26-36-37/39-16
- Risk Codes 10-36/37/38-22-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 1-Bromo-2-fluoro-5-(trifluoromethyl)benzene;1-Bromo-2-fluorobenzene;1-Fluoro-2-bromobenzene;2-Bromo-1-fluorobenzene;2-Bromophenyl fluoride;2-Fluorobromobenzene;2-Fluorophenyl bromide;NSC59696;o-Bromofluorobenzene;o-Fluorobromobenzene;
- PSA 0.00000
- LogP 2.58820
Synthetic route

| Conditions | Yield |
|---|---|
| 90% |
-
-
1202186-81-9
(2-fluorophenyl)(mesityl)iodonium trifluoromethanesulfonate

-
-
1072-85-1
o-fluorobromobenzene

| Conditions | Yield |
|---|---|
| With copper(I) bromide In acetonitrile at 80℃; for 2h; | 71% |

| Conditions | Yield |
|---|---|
| With lead(IV) acetate; trifluoroacetic acid; potassium bromide at 25℃; for 0.5h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With manganese triacetate; trifluoroacetic acid; potassium bromide for 72h; Product distribution; Ambient temperature; Co(CH3COO)3, 67percent aq. CF3COOH, 15 - 10 h; | |
| With potassium nitrate; potassium bromide In water; trifluoroacetic acid at 20℃; for 10h; Product distribution; under argon; |
-
-
462-06-6
fluorobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1435-53-6
2,4-dibromo-1-fluorobenzene

-
C
-
3925-78-8
2,4,6-Tribromofluorobenzene

-
D
-
359-90-0
1-Fluoro-1,2,3,4,5,6-hexabromocyclohexane

-
E
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With bromine In trichlorofluoromethane for 42h; Irradiation; other compounds: o-, m-, p-difluorobenzene; |

-
-
462-06-6
fluorobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1435-53-6
2,4-dibromo-1-fluorobenzene

-
C
-
359-90-0
1-Fluoro-1,2,3,4,5,6-hexabromocyclohexane

-
D
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With bromine In trichlorofluoromethane for 42h; Irradiation; Yield given. Further byproducts given. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
108-86-1
bromobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1073-06-9
3-fluorobromobenzene

-
C
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With fluorine In trichlorofluoromethane at -78℃; for 1h; | A 23 % Chromat. B 17 % Chromat. C 60 % Chromat. |
| With fluorine In trichlorofluoromethane at -78℃; for 1h; Rate constant; Product distribution; competitive reaction with benzene; | A 23 % Chromat. B 17 % Chromat. C 60 % Chromat. |
| With xenon difluoride; boron trifluoride diethyl etherate In acetonitrile at -35 - 20℃; for 2.5h; Inert atmosphere; Overall yield = 56 %; |


-
-
1072-85-1
o-fluorobromobenzene

| Conditions | Yield |
|---|---|
| With potassium fluoride In N,N-dimethyl-formamide at 80℃; for 3h; Yield given; |

| Conditions | Yield |
|---|---|
| With bromobenzene; Cu-HZSM-5 zeolite at 399.9℃; Mechanism; | 11.2 % Chromat. |

-
-
1072-85-1
o-fluorobromobenzene

-
-
1072-85-1
o-fluorobromobenzene

| Conditions | Yield |
|---|---|
| Leiten durch ein Quarz-Rohr bei Temperaturen von 260grad bis 680grad; | |
| Leiten durch ein Pyrex-Rohr bei Temperaturen von 425grad bis 475grad; |
-
-
462-06-6
fluorobenzene

-
-
7726-95-6
bromine

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| at 100℃; |

-
-
75-15-0
carbon disulfide

-
-
462-06-6
fluorobenzene

-
-
7726-95-6
bromine

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1073-06-9
3-fluorobromobenzene

-
C
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| at 55℃; Product distribution; |

-
-
56-23-5
tetrachloromethane

-
-
462-06-6
fluorobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1073-06-9
3-fluorobromobenzene

-
C
-
352-34-1
4-fluoro-1-iodobenzene

-
D
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| Product distribution; |

-
-
462-06-6
fluorobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1073-06-9
3-fluorobromobenzene

-
C
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With bromine at 25℃; for 16h; Irradiation; Yield given; |


| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; sodium methylate In methanol; water; acetonitrile at 40℃; for 4h; | 98 % Chromat. |
-
-
108-86-1
bromobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1000995-64-1
2-bromo-1-oxypentafluorosulfanylbenzene

-
C
-
344455-90-9
4-bromo-1-oxypentafluorosulfanylbenzene

-
R
-
1073-06-9
3-fluorobromobenzene

-
S
-
108-36-1
1,3-dibromobenzene

-
T
-
106-37-6
1.4-dibromobenzene

-
U
-
460-00-4
1-Bromo-4-fluorobenzene

-
V
-
583-53-9
1,2-dibromobenzene

| Conditions | Yield |
|---|---|
| With bis(pentafluorosulfur) peroxide at 100℃; for 17h; |

-
-
108-86-1
bromobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1000995-64-1
2-bromo-1-oxypentafluorosulfanylbenzene

-
C
-
344455-90-9
4-bromo-1-oxypentafluorosulfanylbenzene

-
R
-
1073-06-9
3-fluorobromobenzene

-
S
-
108-36-1
1,3-dibromobenzene

-
T
-
106-37-6
1.4-dibromobenzene

-
U
-
460-00-4
1-Bromo-4-fluorobenzene

-
V
-
583-53-9
1,2-dibromobenzene

| Conditions | Yield |
|---|---|
| With bis(pentafluorosulfur) peroxide In dichloromethane at 150℃; for 22h; |

-
-
128254-27-3
2-Fluoro-1,4-bis-trimethylsilanyl-benzene

-
-
1072-85-1
o-fluorobromobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Br2 2: KF / dimethylformamide / 3 h / 80 °C View Scheme |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride; sodium nitrite |

-
-
1072-85-1
o-fluorobromobenzene

| Conditions | Yield |
|---|---|
| With tetrafluoroboric acid diethyl ether In tetradecafluorohexane at 100℃; for 1h; Balz-Schiemann reaction; Autoclave; solid phase reaction; | 52 %Chromat. |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver(I) acetate In acetonitrile at 100℃; for 24h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 3-chloro-benzenecarboperoxoic acid 2: copper(I) bromide / acetonitrile / 2 h / 80 °C View Scheme |
-
-
108-86-1
bromobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
399-94-0
2-bromo-1,4-difluorobenzene

-
C
-
1073-06-9
3-fluorobromobenzene

-
D
-
348-61-8
1-bromo-3,4-difluorobenzene

-
E
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With xenon difluoride; boron trifluoride diethyl etherate at 0 - 25℃; Cooling with ice; | A 0.06 mmol B 0.01 mmol C 0.03 mmol D 0.004 mmol E 0.16 mmol |


| Conditions | Yield |
|---|---|
| With 2-chloro-[1,10]phenanthroline; {Pd(2,2':6',2''-terpyridine)(acetonitrile)}(BF4)2; Selectfluor In acetonitrile at 50℃; for 12h; Reagent/catalyst; Temperature; | |
| With [(terpy)Pd(2-Cl-phen)][BF4]; Selectfluor In acetonitrile at 50℃; Overall yield = 49 %Spectr.; |
-
-
244205-40-1
(2-bromophenyl)boronic acid

-
-
1072-85-1
o-fluorobromobenzene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: potassium fluoride / acetonitrile / 16 h / 90 °C 2: 2,6-dichloro-1-fluoropyridin-1-ium tetrafluoroborate / chloroform / 6 h / 60 °C View Scheme |

-
-
1072-85-1
o-fluorobromobenzene

| Conditions | Yield |
|---|---|
| With 2,6-dichloro-1-fluoropyridin-1-ium tetrafluoroborate In chloroform at 60℃; for 6h; | 74 %Spectr. |

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromobenzoic-acid With tetrakis(acetonitrile)copper(I)tetrafluoroborate; tetrabutylammonium tetra(tert-butyl alcohol) coordinate fluoride; copper(II) bis(trifluoromethanesulfonate) In acetonitrile at 23℃; for 0.5h; Sealed tube; Inert atmosphere; Stage #2: In acetonitrile at 35℃; for 6h; Irradiation; Sealed tube; Inert atmosphere; | 85 %Spectr. |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 20℃; for 16h; | 100% |
| With sulfuric acid; nitric acid In water at 10 - 20℃; | 89% |
| With sulfuric acid; nitric acid at 10 - 20℃; | 89% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
22988-70-1
6-amino-2,3,5-trimethylbenzo[b]thiophene

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In toluene at 100℃; for 21h; Buchwald-Hartwig coupling; | 100% |
| With palladium diacetate; caesium carbonate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In toluene at 100℃; for 21h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; palladium diacetate; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In toluene for 24h; Heating / reflux; | 100% |
| Stage #1: o-fluorobromobenzene; 2-Fluoroaniline With sodium t-butanolate; palladium diacetate; bis[2-(diphenylphosphino)phenyl] ether In toluene for 24h; Heating / reflux; Stage #2: With water In toluene | 91.4% |
| With sodium t-butanolate; palladium diacetate; bis[2-(diphenylphosphino)phenyl] ether In toluene for 24h; Heating / reflux; | 91.4% |
| With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; sodium t-butanolate In toluene at 110℃; for 16h; Buchwald-Hartwig Coupling; | 89% |
| With tri-tert-butyl phosphine; sodium t-butanolate; palladium diacetate In toluene at 90℃; for 16h; | 78% |

| Conditions | Yield |
|---|---|
| With TMS-NEt2; phosphazene base t-Bu-P4 In hexane; N,N-dimethyl-formamide at 100℃; for 48h; | 100% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
685-83-6
bis(diethylamino)chlorophosphine

-
-
1219134-57-2
(ortho-F-C6H4)P(NEt2)2

| Conditions | Yield |
|---|---|
| Stage #1: o-fluorobromobenzene With n-butyllithium In diethyl ether; hexenes at -89 - -85℃; for 3h; Inert atmosphere; Stage #2: bis(diethylamino)chlorophosphine In diethyl ether; hexenes at -100 - 20℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With monophosphine 1,2,3,4,5-pentaphenyl-1'-(di-tert-butylphosphino)ferrocene; (C5H5)2Zr(OC4H8)N(Si(CH3)3)CH(C6H5); bis(dibenzylideneacetone)-palladium(0) In toluene at 80℃; for 16h; Sealed tube; Inert atmosphere; | 99% |
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; cesium fluoride In dimethyl sulfoxide at 100℃; for 1.5h; | 90% |
| Ni-2,2'-dipyridylamine In ethanol at 20℃; Reduction; Electrolysis; | 68% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
363-72-4
Pentafluorobenzene

-
-
41877-27-4
2'-fluoro-2,3,4,5,6-pentafluoro-1,1'-biphenyl

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate; potassium carbonate In Isopropyl acetate at 80℃; for 12h; | 99% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
51-17-2
benzoimidazole

-
-
1198007-13-4
1-(2-bromophenyl)-1H-benzo[d]imidazole

| Conditions | Yield |
|---|---|
| With potassium phosphate In N,N-dimethyl-formamide at 150℃; | 99% |
| With potassium phosphate In N,N-dimethyl-formamide at 190℃; for 1h; Microwave irradiation; | 86% |
| With caesium carbonate In N,N-dimethyl acetamide |

| Conditions | Yield |
|---|---|
| With potassium phosphate In N,N-dimethyl-formamide at 150℃; | 99% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In 1-methyl-pyrrolidin-2-one at 170℃; for 24h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With caesium carbonate; palladium diacetate In toluene at 80℃; for 10h; Suzuki-Miyaura cross-coupling; | 98% |
| With potassium carbonate In ethanol; water at 20℃; for 6h; Suzuki-Miyaura coupling; | 91% |
| With potassium carbonate; bis(η3-allyl)palladium; Tedicyp In xylene at 140℃; for 20h; Suzuki cross-coupling; | 87% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
476429-08-0
tert-butyl α-(2-fluorophenyl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: bromoacetic acid tert-butyl ester With zinc In tetrahydrofuran at 20℃; for 0.166667h; Inert atmosphere; Sealed tube; Stage #2: o-fluorobromobenzene With monophosphine 1,2,3,4,5-pentaphenyl-1'-(di-tert-butylphosphino)ferrocene; bis(dibenzylideneacetone)-palladium(0) In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; Sealed tube; | 98% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
201230-82-2
carbon monoxide

-
-
20680-59-5
3-methylbenzenecarboximidamide hydrochloride

-
-
18818-40-1
2-(3-methylphenyl)-4(3H)-quinazolinone

| Conditions | Yield |
|---|---|
| With palladium diacetate; N-ethyl-N,N-diisopropylamine; catacxium A In N,N-dimethyl acetamide at 140℃; under 7500.75 Torr; for 22h; Inert atmosphere; Autoclave; | 98% |


| Conditions | Yield |
|---|---|
| Stage #1: o-fluorobromobenzene With 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; bis(dibenzylideneacetone)-palladium(0) In 1,4-dioxane Inert atmosphere; Stage #2: 4,7,10-trioxa-1,13-diaminotridecane With sodium t-butanolate In 1,4-dioxane for 8h; Reagent/catalyst; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: o-fluorobromobenzene With n-butyllithium In tetrahydrofuran; hexane at -30℃; Inert atmosphere; Flow reactor; Stage #2: Trimethyl borate In tetrahydrofuran; hexane at -30℃; Inert atmosphere; Flow reactor; | 97% |

| Conditions | Yield |
|---|---|
| With C36H37NP2; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate In toluene at 110℃; for 120h; Temperature; Reagent/catalyst; Inert atmosphere; Schlenk technique; Glovebox; | 97% |
-
-
1072-85-1
o-fluorobromobenzene


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine; sodium t-butanolate In 1,4-dioxane at 130℃; for 12h; Inert atmosphere; | 97% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
75230-49-8
N′-(1-(4-fluorophenyl)ethylidene)-4-methylbenzenesulfonohydrazide

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); di(1-adamantyl)-4-(N,N-dimethyl)amino-phenyl-phosphane trifluoroacetic acid; lithium tert-butoxide In 1,4-dioxane; water at 110℃; Sealed tube; Inert atmosphere; | 97% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
102632-49-5
1,1-bis(1-methyl-2,3,4,5-tetraphenyl-1-silacyclopentadiene)

| Conditions | Yield |
|---|---|
| With magnesium In tetrahydrofuran | 96% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
764-48-7
2-hydroxyethyl vinyl ether

-
-
261966-94-3
2-(2-fluorophenyl)-2-methyl-1,3-dioxolane

| Conditions | Yield |
|---|---|
| With 1,3-bis-(diphenylphosphino)propane; triethylamine; 1-butyl-3-methylimidazolium Tetrafluoroborate; palladium diacetate at 115℃; for 24h; Heck reaction; | 96% |
| With 1,3-bis-(diphenylphosphino)propane; palladium diacetate; triethylamine In ethylene glycol at 145℃; Heck reaction; Inert atmosphere; regioselective reaction; | 77% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 150℃; for 6h; | 96% |
| With caesium carbonate In N,N-dimethyl-formamide at 150℃; for 12h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: o-fluorobromobenzene With n-butyllithium In diethyl ether at -85 - -75℃; for 1h; Stage #2: N,N-dimethylaminodichlorophosphane In diethyl ether at -85 - 20℃; | 95.95% |

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; cesium fluoride In 2-pentanol at 100℃; for 18h; Inert atmosphere; | A 95.5% B 4.5% |
| With palladium; hydroquinone; potassium hydroxide In glycerol at 90℃; for 18h; chemoselective reaction; | A n/a B 71 %Chromat. |

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; sodium hydride at 100℃; for 1h; | 95% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
918630-49-6
1-cyano-2-(5,5-dimethyl-1,3,2-dioxaborinan-2-yl)naphthalene

-
-
918630-57-6
1-cyano-2-(2-fluorophenyl)naphthalene

| Conditions | Yield |
|---|---|
| With potassium carbonate; tetrakis(triphenylphosphine) palladium(0) In ethanol; toluene at 100℃; for 6h; | 95% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
1069-08-5
diethylphosphoramidous dichloride

-
-
1065188-97-7
(ortho-F-C6H4)2P-NEt2

| Conditions | Yield |
|---|---|
| Stage #1: o-fluorobromobenzene With n-butyllithium In diethyl ether; hexane at -85 - -78℃; for 3h; Stage #2: diethylphosphoramidous dichloride In diethyl ether; hexane at -85 - -10℃; | 95% |
| Stage #1: o-fluorobromobenzene With n-butyllithium In diethyl ether; hexane at -85 - -78℃; for 3h; Stage #2: diethylphosphoramidous dichloride In diethyl ether; hexane at -85 - -10℃; | 95% |
| Stage #1: o-fluorobromobenzene With n-butyllithium In diethyl ether; hexane at -85 - -78℃; for 3h; Stage #2: diethylphosphoramidous dichloride In diethyl ether; hexane at -85 - -10℃; | 95% |
| Stage #1: o-fluorobromobenzene With n-butyllithium In diethyl ether; hexane at -85℃; for 3h; Inert atmosphere; Stage #2: diethylphosphoramidous dichloride In diethyl ether; hexane at -85 - -10℃; | 95% |
| Stage #1: o-fluorobromobenzene With n-butyllithium In diethyl ether; hexane at -85 - -78℃; for 3h; Stage #2: diethylphosphoramidous dichloride In diethyl ether; hexane at -85 - -10℃; | 95% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 150℃; for 12h; Inert atmosphere; | 94.7% |
| With caesium carbonate In N,N-dimethyl-formamide at 150℃; for 12h; Inert atmosphere; | 94.7% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
1257220-47-5
12,12-dimethyl-10,12-dihydro-10-azaindeno[2,1-b]fluorene

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide for 6h; Reflux; | 94.7% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
102632-49-5
1,1-bis(1-methyl-2,3,4,5-tetraphenyl-1-silacyclopentadiene)

-
-
102649-09-2
11,11'-Dimethyl-1,8,9,10,1',8',9',10'-octaphenyl-[11,11']bi[11-sila-tricyclo[6.2.1.02,7]undecyl]-2(7),3,5,9,2'(7'),3',5',9'-octaene

| Conditions | Yield |
|---|---|
| With magnesium In tetrahydrofuran | 94% |
-
-
1072-85-1
o-fluorobromobenzene

-
-
115-19-5
2-methyl-but-3-yn-2-ol

-
-
178173-84-7
4-(2-fluoro-phenyl)-2-methyl-but-3-yn-2-ol

| Conditions | Yield |
|---|---|
| With piperidine; tetrakis(triphenylphosphine) palladium(0); triphenylphosphine; copper(I) bromide; lithium bromide In tetrahydrofuran for 0.5h; Heating; | 94% |
| With copper(l) iodide; diisopropylamine; triphenylphosphine; palladium dichloride In N,N-dimethyl-formamide at 60℃; for 24h; | 78% |
2-Bromofluorobenzene Consensus Reports
2-Bromofluorobenzene Specification
The 1-Fluoro-2-bromobenzene, with the CAS registry number 1072-85-1 and EINECS registry number 214-018-3, is also called 2-Bromofluorobenzene. It is a kind of clear colourless to light yellow liquid, and belongs to the following product categories: Aromatic Hydrocarbons (substituted) & Derivatives; Fluorobenzene; Bromine Compounds; Fluorine Compounds; Aryl; C6; Halogenated Hydrocarbons. And the molecular formula of this chemical is C6H4BrF. What's more, it is used as the intermediate of medicine, pesticide and liquid crystal.
The physical properties of 1-Fluoro-2-bromobenzene are as following: (1)ACD/LogP: 2.96; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.95; (4)ACD/LogD (pH 7.4): 2.95; (5)ACD/BCF (pH 5.5): 103.65; (6)ACD/BCF (pH 7.4): 103.65; (7)ACD/KOC (pH 5.5): 964.64; (8)ACD/KOC (pH 7.4): 964.64; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: Å2; (13)Index of Refraction: 1.53; (14)Molar Refractivity: 33.93 cm3; (15)Molar Volume: 109.8 cm3; (16)Polarizability: 13.45×10-24cm3; (17)Surface Tension: 33.8 dyne/cm; (18)Density: 1.593 g/cm3; (19)Flash Point: 50.2 °C; (20)Enthalpy of Vaporization: 37.23 kJ/mol; (21)Boiling Point: 151.5 °C at 760 mmHg; (22)Vapour Pressure: 4.67 mmHg at 25°C.
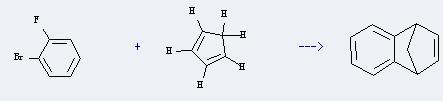
Uses of 1-Fluoro-2-bromobenzene: It can react with cyclopenta-1,3-diene to produce 1,4-dihydro-1,4-methano-naphthalene. This reaction will need reagent Mg, and solvent diethyl ether. The reaction time is 3 hours with the temperature of 20°C, and the yield is about 66%.
You should be cautious while dealing with this chemical. It is a kind of flammale chemical which irritates eyes, respiratory system and skin, and also harmful by inhalation, in contact with skin and if swallowed. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice; Keep away from sources of ignition - No smoking.
You can still convert the following datas into molecular structure:
(1)SMILES: Fc1ccccc1Br
(2)InChI: InChI=1/C6H4BrF/c7-5-3-1-2-4-6(5)8/h1-4H
(3)InChIKey: IPWBFGUBXWMIPR-UHFFFAOYAA
Related Products
- 2-Bromofluorobenzene
- 1072-86-2
- 107289-17-8
- 1072907-44-8
- 1072-91-9
- 1072930-86-9
- 1072-93-1
- 1072944-13-8
- 1072944-19-4
- 1072944-20-7
- 1072944-21-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View