-
Name
2-ETHYLBUTYRALDEHYDE
- EINECS 202-623-5
- CAS No. 97-96-1
- Article Data58
- CAS DataBase
- Density 0.799 g/cm3
- Solubility Insoluble in water, miscible in alcohol and ether
- Melting Point -89oC
- Formula C6H12O
- Boiling Point 118.1 °C at 760 mmHg
- Molecular Weight 100.161
- Flash Point 21.1 °C
- Transport Information
- Appearance clear colorless liquid with cocoa and chocolate aroma
- Safety 16-26-36
- Risk Codes 11-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xi
Xi
- Synonyms Butyraldehyde,2-ethyl- (6CI,7CI,8CI);Butyraldehyde, a-ethyl- (4CI);2-Ethylbutanal;2-Ethylbutyraldehyde;2-Ethylbutyric aldehyde;3-Formylpentane;Diethylacetaldehyde;NSC 6757;a-Ethylbutanal;a-Ethylbutyraldehyde;
- PSA 17.07000
- LogP 1.62150
Synthetic route
-
-
845791-28-8
1,1-diacetoxy-2-ethyl-butane

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| With 2,6-dicarboxypyridinium chlorochromate In acetonitrile at 20℃; for 0.25h; | 96% |
-
-
74947-34-5
N-(Benzylidene)-2-ethyl-1-butenylamine

-
A
-
97-96-1
2-Ethylbutyraldehyde

-
B
-
100-52-7
benzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane for 1h; Heating; | A 93% B n/a |
-
-
143399-67-1
N-(4-Chlorobenzylidene)-2-ethyl-1-butenylamine

-
A
-
97-96-1
2-Ethylbutyraldehyde

-
B
-
104-88-1
4-chlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane for 1h; Heating; | A 87% B n/a |

| Conditions | Yield |
|---|---|
| With phosphoric acid at 100℃; for 3h; | 78% |
-
-
201230-82-2
carbon monoxide

-
-
109-68-2
2-pentene

-
A
-
97-96-1
2-Ethylbutyraldehyde

-
B
-
123-15-9
2-methylvaleraldehyde

-
C
-
66-25-1
hexanal

| Conditions | Yield |
|---|---|
| With hydrogen In methoxybenzene at 120℃; under 37503 Torr; for 6h; Product distribution; Further Variations:; Reagents; Pressures; Temperatures; | A n/a B n/a C 20% |

-
-
64468-90-2
5,5-diethyl-3-nitroso-oxazolidin-2-one

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
63883-69-2
(E)-2-ethyl-2-butenal

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| With ethanol; palladium Hydrogenation; |

| Conditions | Yield |
|---|---|
| With copper chromite at 260℃; |
-
-
66553-16-0
2-ethyl-2-hydroxy-1-butanol

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
155950-22-4
3-ethoxymethyl-pentan-3-ol

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
20521-42-0
2-vinyl-crotonaldehyde

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| With nickel diacetate; iron; acetic acid |
-
-
19780-25-7
2-ethylcrotonaldehyde

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen at 60℃; for 5h; | |
| With nickel Hydrogenation; |

| Conditions | Yield |
|---|---|
| at 110 - 115℃; | |
| at 110 - 115℃; |

| Conditions | Yield |
|---|---|
| at 110 - 115℃; |
-
-
925-90-6
ethylmagnesium bromide

-
-
2315-36-8
2-Chloro-N,N-diethylacetamide

-
A
-
97-96-1
2-Ethylbutyraldehyde

-
B
-
23590-25-2
3-diethylaminomethyl-pentan-3-ol

-
D
-
27794-54-3
N,N-diethyl-2-diethylaminoacetamide


| Conditions | Yield |
|---|---|
| (i) Mg, BrCH2CH2Br, Et2O, (ii) /BRN= 1209224/, (iii) aq. HCl, Et2O; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With perchloric acid In benzene | |
| With hydrogen cation | |
| With water; sodium chloride; hydrogenchloride at 25℃; Rate constant; isotope effects investigated; |
-
-
31499-97-5
2-ethyl-N,N-dimethyl-butyramide

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| With lithium diethoxyaluminum hydride In diethyl ether |

| Conditions | Yield |
|---|---|
| In acetonitrile | |
| (i) MeCN, (ii) aq. NaOAc, AcOH; Multistep reaction; |

| Conditions | Yield |
|---|---|
| (i) MeLi, Et2O, THF, CH2Cl2, (ii) /BRN= 635749/, (iii) NaBO3*4H2O; Multistep reaction; | |
| Yield given. Multistep reaction; |
-
-
646-04-8
(E)-pent-2-ene

-
-
201230-82-2
carbon monoxide

-
A
-
97-96-1
2-Ethylbutyraldehyde

-
B
-
123-15-9
2-methylvaleraldehyde

| Conditions | Yield |
|---|---|
| With hydrogen; cobaltcluster at 150℃; under 51154.1 - 57829.6 Torr; for 22.5h; Mechanism; Product distribution; determination of selectivity, velocity and decomposition of cluster; also with phosphanes; other temperatures, pressures and times; | |
| With hydrogen; acetylacetonatodicarbonylrhodium(l) In dichloromethane at 120℃; under 15514.9 Torr; for 16h; |


| Conditions | Yield |
|---|---|
| In water at 20℃; Rate constant; Thermodynamic data; ΔH++, ΔS++; | |
| In water at 19.9℃; Rate constant; pH = 4 - 5; |
-
-
57240-62-7
2-chloro-2-ethylbutyraldehyde

-
-
100-46-9
benzylamine

-
A
-
97-96-1
2-Ethylbutyraldehyde

-
B
-
100-52-7
benzaldehyde

| Conditions | Yield |
|---|---|
| multistep reaction; other substrates; |


| Conditions | Yield |
|---|---|
| With acetylacetonatodicarbonylrhodium(l); hydrogen; (R,S)-binaphos In benzene at 60℃; under 76000 Torr; for 40h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With acetylacetonatodicarbonylrhodium(l); hydrogen; (R,S)-binaphos In benzene at 60℃; under 76000 Torr; for 40h; Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With acetic acid; quinolinium chlorochromate(VI) at 19.85℃; Kinetics; Further Variations:; Temperatures; Oxidation; |

| Conditions | Yield |
|---|---|
| With 1-penten; η5-pentamethylcyclopentadienylbis(η2-propene)rhodium(I) In benzene at 80℃; for 5h; Isomerization; |

| Conditions | Yield |
|---|---|
| at 110 - 115℃; |
-
-
60-29-7
diethyl ether

-
-
109-68-2
2-pentene

-
A
-
97-96-1
2-Ethylbutyraldehyde

-
B
-
123-15-9
2-methylvaleraldehyde

| Conditions | Yield |
|---|---|
| at 125℃; under 220652 Torr; |


| Conditions | Yield |
|---|---|
| at 125℃; under 220652 Torr; |

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| With phenyldimethylsilyl chloride; zinc; bis(cyclopentadienyl)vanadium dichloride In tetrahydrofuran at 20℃; for 13h; Reduction; | 100% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
74067-45-1
2-amino-5-chloro-α-(2'-chlorophenyl)benzyl alcohol

-
-
152906-78-0
[5-chloro-2-(2-ethyl-butylamino)-phenyl]-(2-chloro-phenyl)-methanol

| Conditions | Yield |
|---|---|
| With sodium cyanoborohydride; acetic acid In methanol at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Ethylbutyraldehyde; diethylzinc With (Sp,S)-5-[C6H11-CH(Me)-N=C(Me)]-4-OH-[2.2]paracyclophane In hexane at 0℃; for 14h; Stage #2: acetic anhydride In hexane at 20℃; for 24h; Further stages.; | 100% |


| Conditions | Yield |
|---|---|
| Stage #1: 4-Phenyl-1-butyne With zinc trifluoromethanesulfonate; triethylamine; (1S,2S)-2-N,N-dimethylamino-1-(p-nitrophenyl)-3-(tert-butyldimethylsilyloxy)propan-1-ol In toluene for 0.25h; Stage #2: 2-Ethylbutyraldehyde In toluene at 25℃; for 2h; Further stages.; | 99% |
| With (difluoromethanesulfonyloxy)zincio difluoromethanesulfonate; triethylamine; (1S,2S)-2-N,N-dimethylamino-1-(p-nitrophenyl)-3-(tert-butyldimethylsilyloxy)propan-1-ol In toluene at 25℃; for 2h; | 83% |

| Conditions | Yield |
|---|---|
| Stage #1: phenylacetylene With zinc trifluoromethanesulfonate; triethylamine; (1S,2S)-2-N,N-dimethylamino-1-(p-nitrophenyl)-3-(tert-butyldimethylsilyloxy)propan-1-ol In toluene for 0.25h; Stage #2: 2-Ethylbutyraldehyde In toluene at 25℃; for 2h; Further stages.; | 99% |
| With (difluoromethanesulfonyloxy)zincio difluoromethanesulfonate; triethylamine; (1S,2S)-2-N,N-dimethylamino-1-(p-nitrophenyl)-3-(tert-butyldimethylsilyloxy)propan-1-ol In toluene at 25℃; for 2h; | 89% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
1066-54-2
trimethylsilylacetylene

-
-
103-49-1
dibenzylamine

-
-
654678-64-5
(-)-N,N-dibenzyl-4-ethyl-1-(trimethylsilyl)hex-1-yn-3-amine

| Conditions | Yield |
|---|---|
| With (R)-1-(2-(diphenylphosphanyl)naphthalen-1-yl)isoquinoline; 4 A molecular sieve; copper(I) bromide In toluene at 20℃; | 99% |
| With (R)-1-(2-(diphenylphosphanyl)naphthalen-1-yl)isoquinoline; 4 A molecular sieve; copper(I) bromide In decane; toluene at 20℃; for 144h; | 99% |
| With (R)-1-(2-(diphenylphosphanyl)naphthalen-1-yl)isoquinoline; copper(I) bromide In toluene at 20℃; | 95% |

-
-
97-96-1
2-Ethylbutyraldehyde

-
-
1066-54-2
trimethylsilylacetylene

-
-
103-49-1
dibenzylamine

-
-
780782-31-2
(+/-)-N,N-dibenzyl-4-ethyl-1-(trimethylsilyl)hex-1-yn-3-amine

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; copper(I) bromide In decane; toluene at 20℃; | 99% |
| With 4 A molecular sieve; copper(I) bromide In toluene at 20℃; | 99% |
| With decane; 4 A molecular sieve; copper(I) bromide In toluene at 20℃; | 86% |


| Conditions | Yield |
|---|---|
| With phenylsilane; pyridine N-oxide; dibutyltin chloride In tetrahydrofuran at 20℃; for 20h; | 99% |

| Conditions | Yield |
|---|---|
| With silica gel In ethanol at 20℃; Ultrasound irradiation; | 99% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
941-69-5
N-phenyl-maleimide

-
-
1322611-71-1
(R)-2-(2,5-dioxo-1-phenylpyrrolidin-3-yl)-2-ethylbutanal

| Conditions | Yield |
|---|---|
| With 1-[(1R,2R)-2-aminocyclohexyl]-3-[4-(n-perfluorooctyl)phenyl]-thiourea; benzoic acid In dichloromethane at 20℃; for 168h; asymmetric Michael addition; optical yield given as %ee; enantioselective reaction; | 99% |
| With 1-((1R,2R)-2-aminocyclohexyl)-3-(3,5-bis(trifluoromethyl)phenyl)thiourea; benzoic acid In dichloromethane at 20℃; for 3h; asymmetric Michael addition; optical yield given as %ee; enantioselective reaction; | 95% |
| With 1H-imidazole; 1-[(1S,2S)-2-aminocyclohexyl]-2,3-diisopropylguanidine In water; N,N-dimethyl-formamide at 0℃; for 96h; Michael Addition; enantioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| With tetrabutoxytitanium; water; 2,4-di-tert-butyl-6-({[(1S)-1-(hydroxymethyl)-2-methylpropyl]imino}methyl)phenol In dichloromethane at 20℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With titanium(IV) tetrabutoxide; triethylsilyl chloride In dichloromethane at 20℃; | A n/a B 99% |

-
-
97-96-1
2-Ethylbutyraldehyde

-
-
1514-82-5
2-bromo-3,3,3-trifluoropropene

-
-
1147879-99-9
5-ethyl-1,1,1-trifluoro-2-heptyn-4-ol

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-3,3,3-trifluoropropene With n-butyllithium; N-ethyl-N,N-diisopropylamine In tetrahydrofuran; hexane at -78℃; for 0.333333h; Inert atmosphere; Stage #2: 2-Ethylbutyraldehyde In tetrahydrofuran; hexane at -78℃; for 1h; Inert atmosphere; | 99% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
58368-67-5
pentan-3-yl formate

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 1h; | 99% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
55145-34-1
2-ethylbutyl 2-ethylbutanoate

| Conditions | Yield |
|---|---|
| With C18BF16(1-)*C34H53F2NiOP2(1+) In toluene at 20℃; for 1h; Glovebox; Inert atmosphere; | 98% |
| With aluminum ethoxide | |
| With aluminum isopropoxide |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
131721-21-6
1-(diethylphosphono)decan-2-one

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 50℃; for 1h; | 98% |
| With sodium hydride In tetrahydrofuran at 50℃; for 1h; other aldehydes and β-ketophosphonates; variation of conditions; |
-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 12h; | 98% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
110470-34-3, 110548-53-3, 110548-54-4, 112564-56-4
diethyl [1-amine(4-methoxyphenyl)methyl]phosphonate

-
-
699000-76-5
diethyl {[(E)-2-ethyl-1-butylidene]amino}(4-methoxyphenyl)methylphosphonate

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane for 3h; Heating; | 98% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
189180-13-0
[Amino-(4-bromo-phenyl)-methyl]-phosphonic acid diethyl ester

-
-
699000-79-8
diethyl (4-bromophenyl){[(E)-2-ethyl-1-butylidene]amino}methylphosphonate

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane for 3h; Heating; | 98% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
2627-86-3
(S)-1-phenyl-ethylamine

-
-
151-50-8
potassium cyanide

-
-
443991-10-4
(2S)-3-ethyl-2-{[(1S)-1-phenylethyl]amino}pentanenitrile

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water at 20℃; for 72h; Strecker reaction; | 98% |

-
-
940881-02-7
(E)-1-(4-tolylthio)-3,3,3-trifluoroprop-1-ene

-
-
97-96-1
2-Ethylbutyraldehyde

| Conditions | Yield |
|---|---|
| Stage #1: (E)-1-(4-tolylthio)-3,3,3-trifluoroprop-1-ene With n-butyllithium; N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran; hexane at -78℃; Stage #2: 2-Ethylbutyraldehyde In tetrahydrofuran; hexane at -78℃; for 0.25h; Further stages.; | 98% |
| Stage #1: (E)-1-(4-tolylthio)-3,3,3-trifluoroprop-1-ene With N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran at -78 - 20℃; Inert atmosphere; Stage #2: With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.166667h; Inert atmosphere; Stage #3: 2-Ethylbutyraldehyde Further stages; | 98% |

| Conditions | Yield |
|---|---|
| With di-n-butylboryl trifluoromethanesulfonate; N-ethyl-N,N-diisopropylamine In dichloromethane at -78℃; optical yield given as %de; stereoselective reaction; | 98% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
941-69-5
N-phenyl-maleimide

-
-
1350897-04-9
(S)-2-(2,5-dioxo-1-phenylpyrrolidin-3-yl)-2-ethylbutanal

| Conditions | Yield |
|---|---|
| With (S)-3-amino-3-phenylpropanoic acid; caesium carbonate In dichloromethane at 20℃; for 48h; Michael Addition; enantioselective reaction; | 98% |
| Stage #1: 2-Ethylbutyraldehyde With tert-butyl (2S,3R)-2-amino-3-hydroxybutanoate; potassium hydroxide In dichloromethane at 23℃; for 0.0333333h; Michael addition; Stage #2: N-phenyl-maleimide In dichloromethane at 23℃; for 6h; Michael addition; optical yield given as %ee; enantioselective reaction; | 96% |
| With NH2-Phg-(D-Pro)-Gly-Leu-OH In acetonitrile at 20℃; for 72h; Michael Addition; enantioselective reaction; | 95% |
| With (R)-3-phenyl-3-aminopropionic acid; potassium hydroxide In dichloromethane at 20℃; for 48h; Michael Addition; enantioselective reaction; |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
7677-24-9
trimethylsilyl cyanide

-
-
100020-20-0
3-ethyl-2-(trimethylsilyloxy)pentanenitrile

| Conditions | Yield |
|---|---|
| With rasta resin-PPh3BnCl In chloroform at 50℃; for 0.5h; Inert atmosphere; | 97% |
| zinc(II) iodide at 90 - 100℃; for 3h; | 82% |
| With zinc(II) iodide | |
| With potassium carbonate In diethyl ether at 20℃; for 6h; Inert atmosphere; |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
21872-32-2, 21872-33-3, 42412-74-8, 17329-20-3
1-phenylethyl isocyanide

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetrabutylammomium bromide In benzene for 2h; Ambient temperature; | 97% |

| Conditions | Yield |
|---|---|
| With magnesium sulfate; triethylamine In dichloromethane for 1h; Ambient temperature; | 97% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
50917-72-1
aminomethylphosphonic acid diethyl ester

-
-
699000-70-9
diethyl {[(E)-2-ethyl-1-butylidene]amino}methylphosphonate

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane for 3h; Heating; | 97% |
| With magnesium sulfate In dichloromethane Yield given; |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
16814-08-7, 42077-95-2, 42077-97-4
diethyl 1-aminophenylmethylphosphonate

-
-
699000-73-2
diethyl {[(E)-2-ethyl-1-butylidene]amino}(phenyl)methylphosphonate

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane for 3h; Heating; | 97% |
-
-
97-96-1
2-Ethylbutyraldehyde

-
-
1066-54-2
trimethylsilylacetylene

-
-
103-49-1
dibenzylamine

-
-
872357-79-4
N,N-dibenzyl-4-ethyl-1-(trimethylsilyl)-1-heptyn-3-amine

| Conditions | Yield |
|---|---|
| With (R)-1-(2-(diphenylphosphanyl)naphthalen-1-yl)isoquinoline; 4 A molecular sieve; copper(I) bromide In decane; toluene at 20℃; | 97% |


| Conditions | Yield |
|---|---|
| With C36H44N4O4S2; copper(I) bromide In methanol at 20℃; for 36h; Henry reaction; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 97% |
| With 4-methyl-N-[(1R)-2-{[(1S,2S)-2-{[(2R)-2-(4-methylbenzenesulfonamido)-2-phenylethyl]amino}-1,2-diphenylethy]amino}-1-phenylethyl]benzene-1-sulfonamido; copper(II) acetate monohydrate In ethanol at 20℃; for 48h; Henry reaction; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 89% |
| With C32H20N4O2 In methanol at 20℃; for 8h; Reagent/catalyst; Henry Nitro Aldol Condensation; enantioselective reaction; | 89% |
2-Ethylbutyraldehyde Consensus Reports
Reported in EPA TSCA Inventory.
2-Ethylbutyraldehyde Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
2-Ethylbutyraldehyde Specification
The Diethyl acetaldehyde is an organic compound with the formula C6H12O. The IUPAC name of this chemical is 2-ethylbutanal. With the CAS registry number 97-96-1 and EINECS 202-623-5, it is also named as 3-Formylpentane. The classification code is Skin / Eye Irritant. It is clear colorless liquid which is stable, but air sensitive. And it can form explosive mixtures with air. What's more, this chemical is incompatible with strong bases, strong reducing agents, oxidizing agents. When heated to decomposition it emits acrid smoke and fumes. In addition, Diethyl acetaldehyde should be sealed in the container and stored in the cool and dry place which must be away from oxidant.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 1.79; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.78; (4)ACD/LogD (pH 7.4): 1.78; (5)ACD/BCF (pH 5.5): 13.38; (6)ACD/BCF (pH 7.4): 13.38; (7)ACD/KOC (pH 5.5): 222.85; (8)ACD/KOC (pH 7.4): 222.85; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.394; (14)Molar Refractivity: 29.99 cm3; (15)Molar Volume: 125.2 cm3; (16)Polarizability: 11.88×10-24 cm3; (17)Surface Tension: 23.9 dyne/cm; (18)Density: 0.799 g/cm3; (19)Flash Point: 21.1 °C; (20)Enthalpy of Vaporization: 35.63 kJ/mol; (21)Boiling Point: 118.1 °C at 760 mmHg; (22)Vapour Pressure: 16.9 mmHg at 25°C.
Preparation of Diethyl acetaldehyde: It can be obtained by the condensation of diethylcarbinol and anhydrous oxalic acid or sulfuric acid.
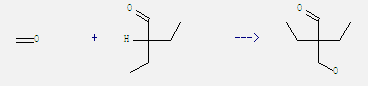
Uses of Diethyl acetaldehyde: It is a kind of spice. And it is mainly used in the preparation of cocoa and chocolate flavor. Besides, it can react with formaldehyde to get 2-ethyl-2-hydroxymethyl-butyraldehyde. This reaction needs reagent Et3N and solvent H2O by heating. The reaction time is 3 hours. The yield is 68%.
When you are using this chemical, please be cautious about it as the following:
It is highly flammable, so people should keep it away from sources of ignition. It is also irritating to eyes, respiratory system and skin. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you want to contact this product, you must wear suitable protective clothing.
People can use the following data to convert to the molecule structure.
1. SMILES:O=CC(CC)CC
2. InChI:InChI=1/C6H12O/c1-3-6(4-2)5-7/h5-6H,3-4H2,1-2H3
3. InChIKey:UNNGUFMVYQJGTD-UHFFFAOYAY
The following are the toxicity data which has been tested.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 5990uL/kg (5.99mL/kg) | Union Carbide Data Sheet. Vol. 12/14/1971, | |
| rat | LCLo | inhalation | 8000ppm/4H (8000ppm) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 4, Pg. 119, 1951. | |
| rat | LD50 | oral | 3980mg/kg (3980mg/kg) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 4, Pg. 119, 1951. |
Related Products
- 2-Ethylbutyraldehyde
- 97963-62-7
- 97966-00-2
- 97966-01-3
- 97966-29-5
- 97968-80-4
- 97970-92-8
- 97-97-2
- 97984-63-9
- 97985-66-5
- 97986-06-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View