-
Name
O-TOLUAMIDE
- EINECS 208-427-6
- CAS No. 527-85-5
- Article Data154
- CAS DataBase
- Density 1.086 g/cm3
- Solubility insoluble in water
- Melting Point 141-142 °C
- Formula C8H9NO
- Boiling Point 254.3 °C at 760 mmHg
- Molecular Weight 135.166
- Flash Point 107.6 °C
- Transport Information
- Appearance white powder
- Safety
- Risk Codes 22
-
Molecular Structure
- Hazard Symbols Xn
- Synonyms Methyl-benzamide;o-Tolylformamide;
- PSA 43.09000
- LogP 1.79420
Synthetic route

| Conditions | Yield |
|---|---|
| With C18H57O3P6Ru2(1+)*C6H5O(1-)*C6H6O; water In 1,4-dioxane for 24h; Time; Sealed tube; Inert atmosphere; Schlenk technique; | 99% |
| With sodium hydroxide; dihydrogen peroxide; tetra(n-butyl)ammonium hydrogensulfate In dichloromethane for 1.6h; Ambient temperature; | 97% |
| With water; sodium carbonate; tricyclohexylphosphine; {Rh(OMe)(cod)}2 In isopropyl alcohol at 25℃; for 24h; | 96% |
-
-
20045-96-9
o-methylbenzoyl bromide

-
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| Stage #1: o-methylbenzoyl bromide With aluminum isopropoxide; dimethyl amine In Cyclooctan at 36℃; for 3h; Stage #2: With potassium sulfate; zinc 2-ethylhexanoate at 47℃; for 3.16667h; Temperature; | 98.8% |

| Conditions | Yield |
|---|---|
| Stage #1: ortho-methylbenzoic acid With 1,3,5-trichloro-2,4,6-triazine; potassium carbonate In tetrahydrofuran for 0.0166667h; Milling; Stage #2: With ammonium thiocyanate In tetrahydrofuran for 0.0833333h; Milling; | 98% |
| With ammonium hydroxide In ethyl acetate at 40℃; for 16h; Solvent; Temperature; Reagent/catalyst; Large scale; Green chemistry; | 97.7% |
| Multi-step reaction with 2 steps 1: phosphorus pentachloride / 120 °C View Scheme |
-
-
14683-79-5
salicylaldoxime

-
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With C55H45ClN5P2Ru(1+)*Cl(1-) In water at 110℃; for 12h; Sealed tube; | 98% |
| With [PdCl2{κ2-(P,N)-2-Ph2PC6H4CH=NOH}] In water at 100℃; for 24h; Inert atmosphere; Sealed tube; | 90% |
| With [RuCl2(η2-C6H6){P(NMe2)3}]; water at 100℃; for 7h; Inert atmosphere; Sealed tube; | 87% |
| With cis,cis,trans-[RuCl2{κ2-(P,N)-2-Ph2PC6H4CH=NOH}2] In water at 100℃; for 12h; Sealed tube; Inert atmosphere; | 82% |
| With zinc(II) chloride In n-heptane for 18h; Reflux; |

| Conditions | Yield |
|---|---|
| With [1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride; silver trifluoroacetate; phenylhydrazine; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In N,N-dimethyl-formamide at 70℃; for 12h; Reagent/catalyst; Solvent; | 96.6% |
| With tert.-butylhydroperoxide; ammonia; oxygen In water; N,N-dimethyl-formamide at 80℃; under 1520.1 Torr; for 5h; Green chemistry; | 89% |
| With tert.-butylhydroperoxide; ammonium hydroxide In water at 100℃; for 16h; | 87% |
| With tert.-butylhydroperoxide; tetraethylammonium iodide; ammonium bicarbonate In 1,2-dichloro-ethane at 70℃; for 22h; | 69% |

| Conditions | Yield |
|---|---|
| With hydroxylamine; C33H28F3N5O3Ru(1+)*Cl(1-) In water at 60℃; for 0.5h; Catalytic behavior; Sonication; | 96% |
| With C17H17BrClN2RuSe(1+)*F6P(1-); hydroxylamine hydrochloride; sodium hydroxide In toluene at 100℃; for 12h; Reagent/catalyst; Solvent; Temperature; | 94% |
| With tert.-butylhydroperoxide; titanium superoxide; saccharin In 1,4-dioxane; hexane at 90℃; for 1h; Green chemistry; | 91% |

| Conditions | Yield |
|---|---|
| With (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); potassium tert-butylate In tert-butyl alcohol at 70℃; for 12h; | 95% |
| With tetra(n-butyl)ammonium hydroxide In water at 70℃; for 12h; Green chemistry; | 89% |
| With potassium tert-butylate; C36H24N2O12Ru3 In tert-butyl alcohol for 10h; Reflux; | 83% |
-
-
933-88-0
ortho-toluoyl chloride

-
-
58-61-7
adenosine

-
A
-
628-13-7
pyridine hydrochloride

-
B
-
104579-36-4
N6,2',3',5'-tetra-O-toluoyladenosine

-
C
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With methanol; ammonia In pyridine at 0℃; | A n/a B 93% C n/a |


| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; N-methoxylamine hydrochloride; sodium iodide; palladium dichloride In acetonitrile at 90℃; under 3800.26 Torr; for 8h; Autoclave; Inert atmosphere; | 93% |
| With 1,4-diaza-bicyclo[2.2.2]octane; palladium 10% on activated carbon; ammonium carbamate; potassium iodide In acetonitrile at 90℃; for 8h; Autoclave; Green chemistry; | 88% |
| With dichloro bis(acetonitrile) palladium(II); ammonium bicarbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In acetonitrile at 120℃; under 7500.75 Torr; for 3h; Autoclave; | > 99 %Chromat. |

| Conditions | Yield |
|---|---|
| With palladium diacetate; ammonium bicarbonate; dicyclohexyl-carbodiimide; triphenylphosphine In acetonitrile at 100℃; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With phosphate buffer at 30℃; for 5.5h; rhodococcus rhodocrous AJ270, pH 7.0; | A 7% B 92% |
| With potassium phosphate buffer at 30℃; for 5.5h; Rhodococcus sp. AJ270 cells; | A 6.6% B 92.4% |
-
-
577-16-2, 122382-54-1
2-Methylacetophenone

-
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With ammonia; water; iodine In tetrahydrofuran at 20℃; for 1h; Haller-Bauer reaction; | 92% |
| With tert.-butylhydroperoxide; ammonia; tetra-(n-butyl)ammonium iodide In water at 100℃; for 16h; Green chemistry; | 51% |
-
-
127459-91-0
(Z)-2-methylbenzaldoxime

-
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With aluminum oxide; methanesulfonic acid at 140℃; for 3h; Beckmann rearrangement; | 90% |

| Conditions | Yield |
|---|---|
| With water; potassium carbonate In dimethyl sulfoxide at 110℃; for 12h; | 90% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); (2R)-1-[(1R)-1-[bis(1,1-dimethylethyl)phosphino]ethyl]-2-(diphenylphosphino)ferrocene; ammonium carbamate; sodium hydrogencarbonate In 1,4-dioxane at 100℃; for 20h; Inert atmosphere; | 86% |
| With 2-(2'-pyridyl)(di(1-adamantyl)phosphino)benzene; ammonia; palladium diacetate In 1,4-dioxane at 120℃; under 1500.15 Torr; for 16h; Autoclave; | 70% |
| With ammonia; palladium diacetate; catacxium A In 1,4-dioxane at 100℃; under 3000.3 Torr; for 16h; Autoclave; | 90 %Chromat. |
| With 1,1'-bis-(diphenylphosphino)ferrocene; ammonia; palladium diacetate In 1,4-dioxane at 100℃; under 1500.15 Torr; for 16h; | 98 %Chromat. |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; 1,8-diazabicyclo[5.4.0]undec-7-ene; N-ethyl-N,N-diisopropylamine; trans-di(μ-acetato)bis[o-(di-o-tolylphosphino)benzyl]dipalladium(II); tri tert-butylphosphoniumtetrafluoroborate In 1,4-dioxane at 110℃; for 0.333333h; microwave irradiation; | 84% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; oxygen In tert-Amyl alcohol at 110℃; under 1500.15 Torr; for 16h; | A 82.8% B 10.4% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; 1,8-diazabicyclo[5.4.0]undec-7-ene; N-ethyl-N,N-diisopropylamine; trans-di(μ-acetato)bis[o-(di-o-tolylphosphino)benzyl]dipalladium(II); tri tert-butylphosphoniumtetrafluoroborate In 1,4-dioxane at 150℃; for 0.333333h; microwave irradiation; | 78% |
-
-
1628342-24-4
(R)-2-amino-3-(((2-methylbenzoyl)carbamothioyl)thio)propanoic acid

-
A
-
98169-56-3
(4R)-2-sulfanylidene-1,3-thiazolidine-4-carboxylic acid

-
B
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| In water for 1h; Reflux; | A n/a B 76% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iron(III) chloride hexahydrate; tetrabutylammonium iodide; ammonium hydroxide In water at 80℃; for 18h; Sealed tube; | 76% |

| Conditions | Yield |
|---|---|
| With copper diacetate; palladium diacetate In water at 120℃; for 1h; Reagent/catalyst; Schlenk technique; Green chemistry; | A 48% B 75% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; ammonia; iodine In water at 100℃; for 3h; Sealed tube; Green chemistry; | 73% |

| Conditions | Yield |
|---|---|
| With [Ru(η6-C6Me6)Cl2(tris(dimethylamino)phosphine)]; hydroxylamine hydrochloride; sodium hydrogencarbonate In water at 100℃; for 7h; Inert atmosphere; Sealed tube; Green chemistry; | A n/a B 71% |
-
-
22364-68-7
2-tolylmethylnitrile

-
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; ammonium hydroxide; iodine In acetonitrile at 70℃; for 36h; Sealed tube; | 70% |
| With copper(I) oxide; oxygen; ammonium chloride; sodium hydroxide In acetonitrile at 120℃; under 760.051 Torr; for 48h; Schlenk technique; | 35% |
-
-
146448-53-5
N-<(4-Methylphenyl)sulfonyl>-2-methylbenzenecarboxamide

-
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide In N,N-dimethyl-formamide at 20℃; | 69% |

| Conditions | Yield |
|---|---|
| With ammonia Inert atmosphere; | 62% |
| With ammonium hydroxide; diethyl ether | |
| With ammonium hydroxide In tetrahydrofuran at 20℃; for 2h; |
-
-
32622-24-5
N-phenyl-2-(2-methylphenyl)formamidine

-
-
100-51-6
benzyl alcohol

-
A
-
758640-21-0
N-Benzylaniline

-
B
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In toluene at 120℃; for 15h; Schlenk technique; Glovebox; Inert atmosphere; Sealed tube; | A 62 %Chromat. B 58% |


-
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With ammonium carbonate In dimethyl sulfoxide at 25℃; for 6h; | 51% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; ammonia In water at 105℃; for 16h; | 50% |

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione; silver(I) acetate In N,N-dimethyl-formamide for 12h; Ambient temperature; or Hg(OAc)2, NBS-Hg(OAc)2; | 100% |
| With tetra-N-butylammonium tribromide; 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane for 1.5h; Ambient temperature; | 97% |
| With sodium bromide In acetonitrile at 50℃; for 6h; Hofmann Rearrangement; Electrochemical reaction; Green chemistry; | 93% |

| Conditions | Yield |
|---|---|
| With iodobenzene; oxone; water In acetonitrile at 20℃; | 100% |
| With sodium hydrogen sulfate; [bis(acetoxy)iodo]benzene In water; acetonitrile at 20℃; for 0.583333h; | 85% |

| Conditions | Yield |
|---|---|
| With per-rhenic acid In water; 1,3,5-trimethyl-benzene for 24h; Heating; | 99% |
| With per-rhenic acid In water; 1,3,5-trimethyl-benzene for 24h; Heating; | 97% |
| With iron(II) chloride tetrahydrate; N-methyl-N-trimethylsilyl-2,2,2-trifluoroacetamide In tetrahydrofuran at 70℃; for 2h; | 96% |
-
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide; trifluoroacetic acid at 20℃; for 3h; regioselective reaction; | 99% |
-
-
527-85-5
o-Methylbenzamid

-
-
76503-37-2
3-amido-4-methylbenzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid at 70℃; for 2h; | 98% |
-
-
603-35-0
triphenylphosphine

-
-
527-85-5
o-Methylbenzamid

-
-
95978-80-6
N-o-toluoyltriphenylphospha-λ5-azene

| Conditions | Yield |
|---|---|
| With diethylazodicarboxylate In tetrahydrofuran at 0 - 25℃; for 12h; | 97% |

| Conditions | Yield |
|---|---|
| With samarium diiodide; water In tetrahydrofuran for 0.00277778h; Ambient temperature; | 96% |
| With C24H20ClN2OPRu; potassium tert-butylate; hydrogen In tetrahydrofuran at 110℃; under 10640.7 Torr; for 36h; Inert atmosphere; Schlenk technique; | 85% |
| With sodium amalgam; ethanol |
-
-
302-17-0
chloral hydrate

-
-
527-85-5
o-Methylbenzamid

-
-
51361-17-2
N-(2,2,2-trichloro-1-hydroxyethyl)-2-methylbenzamide

| Conditions | Yield |
|---|---|
| at 90 - 100℃; | 95% |
| Heating; |
-
-
651768-37-5
6-bromo-2-(2-methoxyethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione

-
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With palladium [2'-(amino-κN)[1,1'-biphenyl]-2-yl-κC][[5-(diphenylphosphino)-9,9-dimethyl-9H-xanthen-4-yl]diphenylphosphine-κP](methanesulfonato-κO); caesium carbonate In 1,4-dioxane at 100℃; for 1.66667h; Inert atmosphere; | 95% |
-
-
91-01-0
1,1-Diphenylmethanol

-
-
527-85-5
o-Methylbenzamid

-
-
10254-11-2
N-(diphenylmethyl)-2-methylbenzamide

| Conditions | Yield |
|---|---|
| With Bromodiphenylmethane In neat (no solvent) at 130℃; for 24h; Temperature; Sealed tube; Green chemistry; | 94% |
| With silica perchloric acid In 1,4-dioxane for 5h; Reflux; | 82% |
-
-
527-85-5
o-Methylbenzamid

-
-
14865-38-4
o-tolylmethanamine monohydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: o-Methylbenzamid With bis(cyclopentadienyl)dihydrozirconium; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane at 20℃; under 760.051 Torr; for 24h; Inert atmosphere; Stage #2: With hydrogenchloride In diethyl ether Inert atmosphere; | 93% |
| Stage #1: o-Methylbenzamid With [(aNHC)KN(SiMe3)2]2; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In toluene at 40℃; for 12h; Inert atmosphere; Glovebox; Schlenk technique; Stage #2: With water; sodium hydroxide In diethyl ether; toluene at 40℃; for 1h; Inert atmosphere; Glovebox; Schlenk technique; Stage #3: With hydrogenchloride In diethyl ether; water Inert atmosphere; Glovebox; Schlenk technique; | 89% |
| Stage #1: o-Methylbenzamid With phenylsilane; C28H18ClMnN2O2; potassium tert-butylate In tetrahydrofuran at 50℃; for 12h; Inert atmosphere; Glovebox; Stage #2: With sodium hydroxide In tetrahydrofuran for 3h; Inert atmosphere; Glovebox; Stage #3: With hydrogenchloride In methanol; diethyl ether Glovebox; Inert atmosphere; | 84% |
| Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With t-BuBrettPhos; water; palladium diacetate; caesium carbonate In tert-butyl alcohol at 110℃; for 2h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; silver carbonate In acetonitrile at 115℃; for 10h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 12h; | 93% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 110℃; for 4h; Inert atmosphere; Sealed tube; | 92% |

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate; dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; copper(II) acetate monohydrate In tert-Amyl alcohol at 20 - 100℃; for 12.33h; Schlenk technique; Sealed tube; regioselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With tri(sec-butyl)borane; (DiMeIHeptCl)Pd(cinammyl)Cl; caesium carbonate In toluene at 80℃; for 24h; Inert atmosphere; Sealed tube; Schlenk technique; | 92% |
2-Methylbenzamide Specification
The Benzamide, 2-methyl-, with the CAS registry number 527-85-5, is also known as Methyl-benzamide and o-Tolylformamide. It belongs to the product categories of AMIDE; Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts. Its EINECS registry number is 208-427-6. This chemical's molecular formula is C8H9NO and molecular weight is 135.1632. What's more, both its IUPAC name and systematic name are the same which is called 2-Methylbenzamide. Its appearance is white powder. It is insoluble in water.
Physical properties about this chemical are: (1)ACD/LogP: 1.20; (2)#of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.2; (4)ACD/LogD (pH 7.4): 1.2; (5)ACD/BCF (pH 5.5): 4.81; (6)ACD/BCF (pH 7.4): 4.81; (7)ACD/KOC (pH 5.5): 107.19; (8)ACD/KOC (pH 7.4): 107.19; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 20.31Å2; (13)Index of Refraction: 1.556; (14)Molar Refractivity: 40 cm3; (15)Molar Volume: 124.4 cm3; (16)Surface Tension: 43.4 dyne/cm; (17)Density: 1.086 g/cm3; (18)Flash Point: 107.6 °C; (19)Enthalpy of Vaporization: 49.17 kJ/mol; (20)Boiling Point: 254.3 °C at 760 mmHg; (21)Vapour Pressure: 0.0174 mmHg at 25 °C; (22)Melting Point: 141-142 °C.
Preparation of Benzamide, 2-methyl-: this chemical can be prepared by 2-Methyl-benzonitrile.
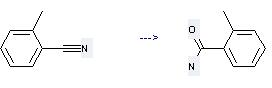
This reaction needs reagents Oxone, Acetone and solvent H2O at temperature of 5-20 °C. The reaction time is 10 hours. The yield is 80%.
Uses of Benzamide, 2-methyl-: it is used to produce other chemicals. For example, it is used to produce 2-Methyl-benzyl alcohol.

The reaction occurs with reagents SmI2, H2O and solvent Tetrahydrofuran. The reaction time is 10 s. The yield is 96%.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(c1ccccc1C)N
(2) InChI: InChI=1/C8H9NO/c1-6-4-2-3-5-7(6)8(9)10/h2-5H,1H3,(H2,9,10)
(3) InChIKey: XXUNIGZDNWWYED-UHFFFAOYAM
Related Products
- 2-Methylbenzamide
- 2-Methylbenzamide oxime
- 52786-55-7
- 52787-14-1
- 52-78-8
- 52788-02-0
- 52788-53-1
- 52789-62-5
- 52789-73-8
- 52791-05-6
- 52793-11-0
- 5279-32-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View