-
Name
3-Pyridinemethanol
- EINECS 202-864-6
- CAS No. 100-55-0
- Article Data158
- CAS DataBase
- Density 1.124 g/cm3
- Solubility soluble in water
- Melting Point -7.7 °C
- Formula C6H7NO
- Boiling Point 266 °C at 760 mmHg
- Molecular Weight 109.128
- Flash Point 97.5 °C
- Transport Information
- Appearance clear light yellow to yellow liquid
- Safety 26-36-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3-(Hydroxymethyl)pyridine;3-Pyridinylmethanol;3-Pyridyl carbinal;3-Pyridylcarbinol;3-Pyridylmethanol;5-(Hydroxymethyl)pyridine;NSC 526046;Nicotinic alcohol;Nu-2121;Pyridine-3-carbinol;RO-1-5155;Roniacol;b-Picolyl alcohol;b-Pyridinecarbinol;b-Pyridylcarbinol;Nicotinyl alcohol;
- PSA 33.12000
- LogP 0.57390
Synthetic route

| Conditions | Yield |
|---|---|
| With LaCu0.67Si1.33; hydrogen In methanol at 120℃; under 22502.3 Torr; for 9h; Autoclave; | 99% |
| With isopropyl alcohol at 300℃; for 3h; | 98% |
| With triethylamine; isopropyl alcohol; lithium bromide at 20℃; for 48h; Meerwein-Ponndorf-Verley reaction; | 97% |

| Conditions | Yield |
|---|---|
| With [RuCl2(N-heterocyclic carbene)(bis[2-(diphenylphosphino)ethyl]amine)]; potassium tert-butylate; hydrogen In tetrahydrofuran at 50℃; under 7500.75 Torr; for 5h; Schlenk technique; Inert atmosphere; | 98% |
| With sodium tetrahydroborate; sodium methylate In methanol at 25℃; for 3h; Reagent/catalyst; Inert atmosphere; | 98% |
| With 2-(Aminomethyl)pyridine; 1,3-bis-(diphenylphosphino)propane; potassium tert-butylate; hydrogen In 2-methyltetrahydrofuran at 100℃; under 37503.8 Torr; for 16h; Reagent/catalyst; Autoclave; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 240℃; for 2h; Autoclave; Inert atmosphere; | 94% |
| With acetic acid; sodium nitrite Diazotization; |

| Conditions | Yield |
|---|---|
| With C30H34Cl2N2P2Ru; potassium methanolate; hydrogen In tetrahydrofuran at 100℃; under 38002.6 - 76005.1 Torr; for 10h; Glovebox; Autoclave; | 90% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 4h; | 87.4% |
| With C39H41FeMnN2O5P(1+)*Br(1-); hydrogen; potassium carbonate In ethanol at 90℃; under 37503.8 Torr; for 16h; | 80% |

| Conditions | Yield |
|---|---|
| With methyloxorhenium(V)(2-(mercaptomethyl)thiophenolate) triphenylphosphine; triphenylphosphine In benzene at 20℃; for 1h; | 90% |
| With 1,1,2,2-tetrabutyl-1,2-dichloro distannane In tetrahydrofuran for 1h; Heating; | 85% |
| With gallium In water for 7h; Heating; | 84% |

| Conditions | Yield |
|---|---|
| With [Zn(BH4)2(py)] In tetrahydrofuran for 0.7h; Heating; | 90% |
| Multi-step reaction with 2 steps 1: Et3N / tetrahydrofuran / 0.5 h / 0 °C 2: Tulusion A-27 exchange resin supporting borohydride anion, Ni(OAc)2 / methanol / 1 h / 10 °C View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid 2: aluminum (III) chloride; sodium tetrahydroborate / tetrahydrofuran; toluene / 0 - 5 °C View Scheme |
-
-
23100-12-1
6-chloronicotinylaldehyde

-
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With hydrogen; sodium acetate; palladium dichloride In methanol at 35℃; under 760.051 Torr; for 2h; | 88% |
-
-
113118-81-3
5-bromopyridine-3-carbaldehyde

-
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With hydrogen; sodium acetate; palladium dichloride In methanol at 35℃; under 760.051 Torr; for 2h; | 83% |

| Conditions | Yield |
|---|---|
| With TEA; magnesium bromide In dichloromethane at 20℃; for 48h; Cannizzaro reaction; | A 82% B n/a |
| With N,N,N',N'-tetramethylguanidine In water at 20℃; for 7h; Cannizzaro reaction; | A 44% B 41% |
| With barium dihydroxide; formaldehyd at 100 - 110℃; for 0.0333333h; Irradiation; | A 97 % Chromat. B 3 % Chromat. |
| With aluminum oxide; sodium hydroxide; water for 0.00416667h; Irradiation; Yield given; Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With bromine; isopentyl nitrite In dichloromethane Product distribution / selectivity; | A 81% B 11% C 3% |
| With bromine; isopentyl nitrite In tetrahydrofuran Product distribution / selectivity; | A 79% B 11% C 3% |
| With bromine; isopentyl nitrite In chloroform Product distribution / selectivity; | A 67% B 18% C 3% |

-
-
500-22-1
3-pyridinecarboxaldehyde

-
-
108460-23-7
5-carbamoyl 4,7-dihydro thieno<2,3-b>pyridine

-
A
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate; water In acetonitrile at 65℃; | A 80% B n/a |
| With magnesium(II) perchlorate; water In acetonitrile at 65℃; | A 80% B n/a |
| With magnesium(II) perchlorate; water In acetonitrile at 65℃; Product distribution; water-sensitivity; |

-
-
149806-06-4
6-bromonicotinaldehyde

-
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With hydrogen; sodium acetate; palladium dichloride In methanol at 35℃; under 760.051 Torr; for 4h; | 78% |

| Conditions | Yield |
|---|---|
| With formaldehyd; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 In water; toluene at 90℃; | A 21% B 74% |
-
-
500-22-1
3-pyridinecarboxaldehyde

-
-
952-92-1
1-Benzyl-1,4-dihydronicotinamide

-
A
-
100-55-0
3-hydroxymethylpyridin

-
B
-
15519-25-2
3-(aminocarbonyl)-1-(phenylmethyl)pyridinium perchlorate

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate In acetonitrile at 65℃; | A 70% B n/a |
| With magnesium(II) perchlorate In acetonitrile at 65℃; Product distribution; |

-
-
6968-72-5
3-pyridylcarbinol-N-oxide

-
A
-
500-22-1
3-pyridinecarboxaldehyde

-
B
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With 1,1'-bis(diphenylphosphino)ferrocene; palladium diacetate; triethylamine In acetonitrile at 150℃; for 1h; Microwave irradiation; chemoselective reaction; | A 20% B 66% |

| Conditions | Yield |
|---|---|
| With C24H20ClN2OPRu; potassium tert-butylate; hydrogen In tetrahydrofuran at 110℃; under 10640.7 Torr; for 36h; Inert atmosphere; Schlenk technique; | 63% |
| With {Ru(H)(BH4)(CO)(3-(di-tert-butylphosphino)-N-((1-methyl-1H-imidazol-2-yl)methyl)propylamine)}; hydrogen In isopropyl alcohol at 150℃; under 37503.8 Torr; for 18h; Autoclave; | 95 %Chromat. |
| With C16H25MnN3O3P(1+)*Br(1-); potassium tert-butylate; hydrogen In tert-Amyl alcohol; cyclohexane at 140℃; under 37503.8 Torr; for 24h; Inert atmosphere; Autoclave; | 55 %Chromat. |

-
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With sodium hydrogen sulfate; eosin; dihydrogen peroxide In water; acetonitrile for 48h; Irradiation; | 57% |

| Conditions | Yield |
|---|---|
| With sodium carbonate; bis(dibenzylideneacetone)-palladium(0); ruphos In 1,4-dioxane; water for 48h; Suzuki-Miyaura cross-coupling; Inert atmosphere; Reflux; | 51.9% |

| Conditions | Yield |
|---|---|
| With bromine; acetic acid; sodium nitrite In dichloromethane Product distribution / selectivity; | A 44% B 49% |
-
-
6968-72-5
3-pyridylcarbinol-N-oxide

-
-
16420-13-6
N,N-Dimethylthiocarbamoyl chloride

-
A
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| In acetonitrile at 81℃; for 4h; | A 47% B 9% |

-
-
500-22-1
3-pyridinecarboxaldehyde

-
-
6290-49-9
methyl methoxyacetate

-
A
-
100-55-0
3-hydroxymethylpyridin

-
B
-
93-60-7
methyl 3-pyridinecarboxylate

-
C
-
136138-66-4
methyl α-methoxy-β-(β-pyridyl)acrylate

| Conditions | Yield |
|---|---|
| With sodium methylate In toluene for 2h; Heating; | A n/a B 10% C 31% |

-
-
626-60-8
3-Chloropyridine

-
A
-
859842-81-2
3-(pivaloxymethyl)pyridine

-
B
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With potassium phosphate; water; dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate In 1,4-dioxane at 20 - 100℃; for 14.25h; | A 12% B 14% |

-
-
500-22-1
3-pyridinecarboxaldehyde

-
-
1342295-75-3
Br(1-)*C9H21N2O(1+)

-
A
-
100-55-0
3-hydroxymethylpyridin

-
B
-
59-67-6
nicotinic acid

| Conditions | Yield |
|---|---|
| Stage #1: Br(1-)*C9H21N2O(1+) With sodium hydroxide In dichloromethane; water at 0℃; for 0.0833333h; Inert atmosphere; Stage #2: 3-pyridinecarboxaldehyde In dichloromethane; water at 0 - 25℃; for 24h; Inert atmosphere; diastereoselective reaction; | A n/a B n/a C 10% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; palladium on activated charcoal Hydrogenation; |

-
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With water Zerlegen des entstandenen 3-Oxymethyl-pyridin-pikrats mit Natronlauge; |

| Conditions | Yield |
|---|---|
| With tin(IV) oxide In isopropyl alcohol at 300℃; for 0.5h; | A 5 % Chromat. B 35 % Chromat. |
-
-
77934-69-1
2-Methyl-benzoic acid pyridin-3-ylmethyl ester

-
A
-
118-90-1
ortho-methylbenzoic acid

-
B
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With water in alkaline medium In acetone Rate constant; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With phosphate buffer In water at 65℃; Rate constant; Thermodynamic data; pH 5.17, ΔS, activation energy, other pH, solvent effect; |
-
-
13469-94-8
3-nitrooxymethylpyridine

-
A
-
500-22-1
3-pyridinecarboxaldehyde

-
B
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 3h; Ambient temperature; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| With 10% Ru/C; oxygen In toluene at 90℃; under 760.051 Torr; for 24h; | 100% |
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In dimethyl sulfoxide for 0.25h; Ambient temperature; | 99% |
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl; oxygen; copper(I) bromide dimethylsulfide complex In chlorobenzene at 80℃; for 8h; | 99% |

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane Reflux; | 100% |
| With thionyl chloride for 0.583333h; Cooling with ice; Reflux; | 72.5% |
| With 1-methyl-pyrrolidin-2-one; benzenesulfonyl chloride In 1,2-dichloro-ethane at 80℃; for 1.5h; | 41% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
222055-82-5
1-(2,2-diethoxyethyl)-4-(imidazol-1-yl)carbonyl-5-aminopyrazole

-
-
222055-89-2
5-amino-1-(2,2-diethoxy-ethyl)-1H-pyrazole-4-carboxylic acid pyridin-3-ylmethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0 - 5℃; for 0.166667h; | 100% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
952596-21-3
C25H28N2O5S

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; | 100% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
953435-15-9
6-methyl 1-pyridin-3-ylmethyl 3,4-dihydroquinoline-1,6(2H)-dicarboxylate

| Conditions | Yield |
|---|---|
| at 20℃; for 8h; | 100% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
760991-59-1
3-iodo-7-methoxy-4,4-dimethyl-1-{[2-(trimethylsilyl)ethoxy]methyl}-1,4-dihydroindeno[1,2-c]pyrazol-6-ol

-
-
760991-63-7
3-iodo-7-methoxy-4,4-dimethyl-6-(pyridin-3-ylmethoxy)-1-{[2-(trimethylsilyl)ethoxy]methyl}-1,4-dihydroindeno[1,2-c]pyrazole

| Conditions | Yield |
|---|---|
| With di-tert-butyl-diazodicarboxylate; Ph3P on solid support In tetrahydrofuran at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 25℃; for 0.05h; Sonication; | 100% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 25℃; for 0.05h; Sonication; | 100% |
-
-
100-55-0
3-hydroxymethylpyridin

| Conditions | Yield |
|---|---|
| With C29H28F6N4OS at 20℃; for 3h; enantioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With 4-N,N-dimethylaminopyridinium saccharinate at 25℃; for 0.5h; Inert atmosphere; Neat (no solvent); | 99% |
| With 4-N,N-dimethylaminopyridinium saccharinate In neat (no solvent) at 25℃; for 0.5h; Reagent/catalyst; | 99% |
| With triethylamine In dichloromethane at 0 - 20℃; for 16h; Inert atmosphere; | 99% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
149882-14-4
10,11-(methylenedioxy)-7-(chloromethyl)-(20S)-camptothecin

| Conditions | Yield |
|---|---|
| for 16h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-hydroxymethylpyridin; methyl iodide at 0℃; for 3h; Stage #2: With silver perchlorate In methanol at 0℃; for 1h; | 99% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
49673-77-0
3-Pyridinecarboxylic acid 3-pyridinylmethyl ester

| Conditions | Yield |
|---|---|
| With iodine; potassium carbonate In tert-butyl alcohol at 20℃; for 19h; | 99% |
| With RuH(CO)Cl(PPh3)(κ2-CP); caesium carbonate In toluene at 110℃; for 26h; Schlenk technique; Glovebox; Inert atmosphere; | 87% |
| With silver(I) hexafluorophosphate; oxygen; palladium diacetate; potassium carbonate; 1,2-bis[di(t-butyl)phosphinomethyl]benzene In toluene at 110℃; under 750.075 Torr; for 20h; chemoselective reaction; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-hydroxymethylpyridin With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tert-butylhypochlorite In dichloromethane at 20℃; for 1h; Inert atmosphere; Stage #2: With ammonia; iodine In dichloromethane; water at 20℃; for 2h; Inert atmosphere; | 99% |
| With ammonia; oxygen In tert-Amyl alcohol; water at 100℃; under 3750.38 Torr; for 4h; Autoclave; High pressure; | 99% |
| With potassium phosphate; ammonium formate In acetonitrile at 115℃; for 16h; Sealed tube; Green chemistry; | 91% |

| Conditions | Yield |
|---|---|
| With tert-butyldimethylsilyl chloride In methanol; water at 20℃; for 0.5h; Reagent/catalyst; Green chemistry; | 99% |
| With 4-acetylamino-2,2,6,6-tetramethyl-1-piperidinoxy In aq. buffer at 20℃; for 12h; pH=9.8 - 10.1; Electrolysis; | 96% |
| With ferrous(II) sulfate heptahydrate; Oxone In water at 20℃; for 0.5h; Sonication; Green chemistry; | 94% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
185346-79-6
1-(benzyloxy)-4-bromo-3-fluorobenzene

-
-
1355091-47-2
3-[5-(benzyloxy)-2-bromophenoxymethyl]pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 3-hydroxymethylpyridin In 1-methyl-pyrrolidin-2-one; mineral oil at 20℃; for 0.5h; Stage #2: 4-(benzyloxy)-1-bromo-2-fluorobenzene In 1-methyl-pyrrolidin-2-one; mineral oil at 100℃; for 0.5h; | 99% |
| Stage #1: 3-hydroxymethylpyridin With sodium hydride In 1-methyl-pyrrolidin-2-one at 20℃; for 0.5h; Stage #2: 4-(benzyloxy)-1-bromo-2-fluorobenzene at 100℃; for 0.25h; |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
98-86-2
acetophenone

-
-
39976-56-2
1-phenyl-3-(pyridin-3-yl)propan-1-one

| Conditions | Yield |
|---|---|
| With titanium(IV) oxide; potassium hydroxide In toluene at 100℃; for 10h; Sealed tube; Irradiation; | 99% |
| With (carbonyl)(chloro)(hydrido)tris(triphenylphosphine)ruthenium(II); caesium carbonate In toluene at 140℃; for 20h; Inert atmosphere; Sealed tube; | 89% |
| With C38H43IrN2PS2(1+)*CF3O3S(1-); caesium carbonate In tert-Amyl alcohol at 120℃; for 24h; Schlenk technique; Inert atmosphere; | 86% |
-
-
462-08-8
pyridin-3-ylamine

-
-
100-55-0
3-hydroxymethylpyridin

-
-
78675-94-2
N-(pyridin-3-ylmethyl)pyridin-3-amine

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 130℃; for 25h; Schlenk technique; Inert atmosphere; | 99% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
74-88-4
methyl iodide

-
-
6457-55-2
3-(Hydroxymethyl)-1-methylpyridinium iodide

| Conditions | Yield |
|---|---|
| In toluene for 1h; Heating; | 98% |
| In dichloromethane at 20℃; for 12h; | 90% |

| Conditions | Yield |
|---|---|
| With TEA In ethyl acetate at 0℃; for 3h; | 98% |
| With triethylamine In dichloromethane at 0 - 25℃; |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
85719-72-8
3-<(trimethylsilyloxy)methyl>pyridine

| Conditions | Yield |
|---|---|
| With potassium fluoride incorporated on clinoptilolite nanoparticles In dichloromethane at 20℃; for 0.666667h; chemoselective reaction; | 98% |
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione In acetonitrile at 20℃; for 0.25h; | 90% |
| With polyvinylpolypyrrolidonium tribromide In acetonitrile at 20℃; for 0.25h; | 60% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; sodium chloride; 1,1'-carbonyldiimidazole In N,N-dimethyl-formamide | 98% |
| Stage #1: 3-hydroxymethylpyridin With 1,1'-carbonyldiimidazole In tetrahydrofuran at 10 - 20℃; for 1h; Stage #2: p-aminomethylbenzoic acid With 1,8-diazabicyclo[5.4.0]undec-7-ene; triethylamine In tetrahydrofuran at 20℃; for 5h; Stage #3: With hydrogenchloride; water pH=5; | 91% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
6089-09-4
4-pentynoic acid

-
-
1268158-83-3
2-pyridinylmethyl 4-pentynoate

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0 - 20℃; | 98% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
91-63-4
2-methylquinoline

-
-
21203-10-1
2-(2-(pyridin-3-yl)ethyl)quinolone

| Conditions | Yield |
|---|---|
| With [Mn(HN(C2H4PiPr2)2)(CO)2Br]; potassium tert-butylate In tert-Amyl alcohol at 140℃; for 24h; Sealed tube; Inert atmosphere; Schlenk technique; Glovebox; | 98% |
| With cesium hydroxide In 5,5-dimethyl-1,3-cyclohexadiene at 160℃; for 24h; | 92% |
| With ruthenium(III) chloride trihydrate; potassium tert-butylate In 1,4-dioxane at 130℃; for 24h; Inert atmosphere; | 65% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
98-86-2
acetophenone

-
-
4452-13-5
1-phenyl-3-(pyridin-3-yl)prop-2-en-1-one

| Conditions | Yield |
|---|---|
| With titanium nitride; potassium hydroxide In toluene at 100℃; for 10h; Sealed tube; Irradiation; | 98% |
-
-
100-55-0
3-hydroxymethylpyridin

-
-
1670-14-0
benzamidine monohydrochloride

-
-
1596320-79-4
2,4-di-phenyl-6-(pyridin-3-yl)-1,3,5-triazine

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) acetate monohydrate; sodium carbonate In toluene at 110℃; for 24h; | 97% |
| With μ-diiodo-di((η5-pentamethylcyclopentadienyl)(iodo)iridium); caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 20 - 110℃; for 20h; Reagent/catalyst; Solvent; | 81% |
| With [RhCl2(p-cymene)]2; caesium carbonate In dimethyl sulfoxide at 110℃; for 16h; Sealed tube; | 78% |
| Stage #1: 3-hydroxymethylpyridin; benzamidine monohydrochloride With caesium carbonate In dimethyl sulfoxide at 20℃; for 0.0833333h; Schlenk technique; Green chemistry; Stage #2: With N-iodo-succinimide In dimethyl sulfoxide at 100℃; for 16h; Schlenk technique; Green chemistry; | 72% |
3-Pyridinemethanol Chemical Properties
Molecular Structure of Nicotinyl alcohol (CAS NO.100-55-0):
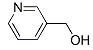
Molecular Formula: C6H7NO
Molecular Weight: 109.14 g/mol
Density: 1.131 g/cm3
Melting Point: -7.7 °C
Boiling Point: 266 °C at 760 mmHg
Flash Point: 97.5 °C
Index of Refraction: 1.551
Molar Refractivity: 30.79 cm3
Molar Volume: 96.4 cm3
Surface Tension: 48.7 dyne/cm
Enthalpy of Vaporization: 53.24 kJ/mol
Vapour Pressure: 0.00442 mmHg at 25 °C
Storage Temp.: 2-8 °C
Water Solubility: soluble
Sensitive: hygroscopic
H-Bond Donor: 1
H-Bond Acceptor: 2
Rotatable Bond Count: 1
Exact Mass: 109.052764
MonoIsotopic Mass: 109.052764
Topological Polar Surface Area: 33.1
Heavy Atom Count: 8
Canonical SMILES: C1=CC(=CN=C1)CO
InChI: InChI=1S/C6H7NO/c8-5-6-2-1-3-7-4-6/h1-4,8H,5H2
InChIKey: MVQVNTPHUGQQHK-UHFFFAOYSA-N
EINECS: 202-864-6
Product Categories: Alkohols
3-Pyridinemethanol Uses
Nicotinyl alcohol (CAS NO.100-55-0) is used as the intermediates of rodenticides in organic synthesis. It has a direct effect on peripheral vascular wall, so it can expand blood vessels and reduce the cholesterol.
3-Pyridinemethanol Toxicity Data With Reference
| 1. | ivn-mus LD50:1000 mg/kg | PHARAT Pharmazie. 11 (1956),242. |
3-Pyridinemethanol Consensus Reports
Reported in EPA TSCA Inventory.
3-Pyridinemethanol Safety Profile
Moderately toxic by intravenous route. When heated to decomposition it emits toxic vapors of NOx.
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 26-36-24/25
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
24/25: Avoid contact with skin and eyes
WGK Germany: 3
RTECS: UT4690000
F: 3-10-23
F3: Hygroscopic
F10: Keep under argon
F23: Sensitive to air
Hazard Note: Irritant
HS Code: 29333999
3-Pyridinemethanol Specification
Nicotinyl alcohol (CAS NO.100-55-0) is also named as 3-(Hydroxymethyl)pyridine ; 3-Picolyl alcohol ; 3-Pyridinylmethanol ; 3-Pyridylcarbinol ; 3-Pyridylmethanol ; 5-21-02-00172 (Beilstein Handbook Reference) ; BRN 0107851 ; NSC 526046 ; NU-2121 ; Nicotinic alcohol ; Pyridine-3-carbinol ; Ro-1-5155 ; Roniacol ; UNII-9TF312056Y ; beta-Picolyl alcohol ; beta-Pyridinecarbinol . Nicotinyl alcohol (CAS NO.100-55-0) is clear light yellow to yellow liquid. It is easily soluble in water and ether.
Related Products
- 3-Pyridinemethanol
- 3-Pyridinemethanol, 2,6-dimethoxy-
- 3-Pyridinemethanol, 2-amino-5-iodo-
- 3-Pyridinemethanol, 4-methyl-
- 3-Pyridinemethanol, 6-chloro-5-(trifluoromethyl)-
- 3-Pyridinemethanol, a,a-bis(4-chlorophenyl)-
- 3-Pyridinemethanol, alpha-(aminomethyl)-
- 3-Pyridinemethanol, a-methyl-, (aS)-
- 3-Pyridinemethanol,2-chloro-5-methyl-
- 3-Pyridinemethanol,4,6-dichloro-
- 100553-83-1
- 100556-58-9
- 1005-56-7
- 100557-22-0
- 100558-60-9
- 100-56-1
- 100563-34-6
- 100563-50-6
- 1005-63-6
- 100564-78-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View