-
Name
4-Iodobiphenyl
- EINECS 216-469-1
- CAS No. 1591-31-7
- Article Data157
- CAS DataBase
- Density 1.584 g/cm3
- Solubility Slightly soluble in water.
- Melting Point 110-114 °C
- Formula C12H9I
- Boiling Point 322.7 °C at 760 mmHg
- Molecular Weight 280.108
- Flash Point 145.8 °C
- Transport Information UN 3077 9/PG 3
- Appearance Off-white crystalline powder
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, N,
N, Xn
Xn
- Synonyms Biphenyl, 4-iodo- (8CI);1, 1-Biphenyl, 4-iodo-;p-Phenyliodobenzene;Biphenyl, 4-iodo-;4-Biphenyl iodide;p-Iodobiphenyl;1,1-Biphenyl, 4-iodo-;1-iodo-4-phenyl-benzene;
- PSA 0.00000
- LogP 3.95820
Synthetic route

| Conditions | Yield |
|---|---|
| With dicloroiodoisocyanuric acid In acetonitrile at 20℃; for 2.5h; Darkness; regioselective reaction; | 99% |
| With sulfuric acid; iodine; periodic acid; H-β zeolite In water; acetic acid at 20 - 118℃; for 6h; Heating / reflux; | 92% |
| With tetramethylammonium dibromoiodate(I) at 20℃; for 0.333333h; | 90% |

| Conditions | Yield |
|---|---|
| With potassium phosphate In ethanol; water at 28℃; for 24h; Catalytic behavior; Suzuki-Miyaura Coupling; Schlenk technique; Inert atmosphere; Green chemistry; | 99% |
| With 5%-palladium/activated carbon; sodium carbonate In water at 60℃; for 6h; Reagent/catalyst; Temperature; | 95% |
| With sodium hydroxide In ethanol at 25℃; for 1.5h; Suzuki-Miyaura Coupling; Irradiation; Sealed tube; Green chemistry; | 92% |
| With potassium carbonate In water at 90℃; for 5h; Suzuki Coupling; Green chemistry; | 91% |
| With 1,4-diaza-bicyclo[2.2.2]octane; copper(l) iodide; tetrabutylammomium bromide; caesium carbonate In N,N-dimethyl-formamide at 130℃; for 24h; Catalytic behavior; Reagent/catalyst; Suzuki Coupling; | 80% |

| Conditions | Yield |
|---|---|
| With sodium nitrite In acetonitrile at 80℃; for 0.5h; Sealed tube; | 99% |
| With N-iodo-succinimide; potassium acetate In acetonitrile at 50℃; for 4h; | 97% |
| With potassium fluoride; iodine In 1,4-dioxane at 80℃; for 1h; | 93% |
| With perfluoroisopropyl iodide; copper; hydroquinone In N,N-dimethyl-formamide at 20℃; for 24h; | 88% |
| With iodine; potassium carbonate In toluene at 70℃; under 750.075 Torr; for 7h; Inert atmosphere; | 50.8% |
-
-
91493-19-5
4-iodoxybiphenyl

-
-
501-65-5
diphenyl acetylene

-
A
-
1591-31-7
4-iodo-biphenyl

-
B
-
134-81-6
benzil

| Conditions | Yield |
|---|---|
| In nitrobenzene at 170℃; for 5h; | A 98% B 34.5% |

-
-
1514-50-7
4-iodobenzenediazonium tetrafluoroborate

-
-
98-80-6
phenylboronic acid

-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With polystyrene-supported-diiodobis(theophylline)palladium(II) complex In methanol at 25℃; for 3h; Suzuki-Miyaura Coupling; chemoselective reaction; | 98% |
| With 5% Pd/BaCO3 In methanol at 25℃; for 12h; | 87% |
| polystyrene-supported N-heterocyclic carbene-Pd complex In ethanol at 20℃; for 12h; Suzuki-Miyaura cross-coupling; | 65% |

-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With trimethylsilyl iodide; methyl iodide In N,N-dimethyl-formamide for 1h; Ambient temperature; | 97% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; Trimethylenediamine; sodium iodide In pentan-1-ol at 130℃; for 22h; aromatic Finkelstein reaction; | 96% |
| Stage #1: 4-bromo-1,1'-biphenyl With Trimethylenediamine; sodium iodide; copper(l) iodide In pentan-1-ol at 130℃; for 22h; Stage #2: With ammonia | 96% |
| With copper(l) iodide; trans-N,N'-dimethylcyclohexane-1,2-diamine; sodium iodide In acetonitrile at 100℃; for 1h; Buchwald's halogen exchange reaction; Inert atmosphere; Microwave irradiation; | 96% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 90℃; for 6h; Hiyama Coupling; Green chemistry; | 93% |
| With sodium hydroxide In ethanol at 25℃; for 5h; Hiyama Coupling; Irradiation; Sealed tube; Green chemistry; | 88% |

| Conditions | Yield |
|---|---|
| palladium diacetate In tetrahydrofuran at 20℃; for 0.5h; Stille reaction; | 90% |

-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With 2-iodo-propane; dimethylglyoxal In water at 23℃; for 24h; Inert atmosphere; Schlenk technique; Irradiation; Green chemistry; | 90% |
| With acetic acid; lithium iodide In dimethyl sulfoxide at 100℃; for 8h; Schlenk technique; | 71% |
| With tetra-(n-butyl)ammonium iodide; copper(II) bis(trifluoromethanesulfonate) In acetonitrile at 25℃; for 16h; Sealed tube; | 68% |
| With caesium tribromide In water; N,N-dimethyl-formamide at 80℃; for 6h; chemoselective reaction; | 45% |

| Conditions | Yield |
|---|---|
| With bis(benzonitrile)palladium(II) dichloride; camphor-10-sulfonic acid; tetrabutyl ammonium fluoride In tetrahydrofuran at 50℃; for 3h; Hiyama Coupling; | 89% |
-
-
144930-55-2
(4-iodophenyl)(phenyl)iodonium trifluoromethanesulfonate

-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With palladium diacetate at 50℃; for 0.166667h; Microwave irradiation; | 89% |

| Conditions | Yield |
|---|---|
| With 1% Pd/C In methanol at 50℃; for 0.5h; Suzuki-Miyaura reaction; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With diamminedichloropalladium(II); copper(II) choride dihydrate; potassium carbonate In water at 25℃; for 12h; Suzuki Coupling; Green chemistry; | 88% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; iodine In ethyl acetate at 60℃; for 16h; | A 12% B 87% |
| With [bis(acetoxy)iodo]benzene; iodine In ethyl acetate at 60℃; for 16h; Iodination; | A 12% B 87% |
| With poly; iodine In ethyl acetate at 60℃; for 16h; | A 43% B 2% |
-
-
3958-47-2
triphenylindium

-
A
-
92-52-4
biphenyl

-
B
-
21650-47-5
(E)-1-(4-iodophenyl)-2-phenyldiazene

-
C
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran at 20℃; for 0.0833333h; | A 6% B 3% C 85% |


| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In tert-butyl methyl ether at 60℃; for 6h; Suzuki Coupling; | 84% |

| Conditions | Yield |
|---|---|
| With 1,3-bis-(diphenylphosphino)propane; potassium acetate; palladium dichloride In dimethyl sulfoxide at 60℃; for 6h; Suzuki-Miyaura Coupling; | 83% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-phenylalanine With sulfuric acid; silica gel; sodium nitrite at 20℃; grinding; Neat (no solvent); Stage #2: With water at 20℃; grinding; Neat (no solvent); Stage #3: With potassium iodide at 20℃; for 0.166667h; Sandmeyer reaction; grinding; Neat (no solvent); | 82% |
| With toluene-4-sulfonic acid; potassium iodide; sodium nitrite In acetonitrile at 20℃; for 0.5h; | 80% |
| Stage #1: 4-phenylalanine With nicotinic acid sulfate; sodium nitrite In water at 20℃; for 0.333333h; Grinding; Stage #2: With sodium iodide In water at 20℃; | 80% |
-
-
5122-94-1
4-biphenylboronic acid

-
-
140-29-4
phenylacetonitrile

-
A
-
2920-38-9
4-cyano-1,1'-biphenyl

-
B
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; copper(l) iodide In N,N-dimethyl acetamide; water at 130℃; for 16h; Reagent/catalyst; Solvent; Temperature; Time; | A 82% B 13% |
| With tert.-butylhydroperoxide; copper(l) iodide In N,N-dimethyl acetamide; water at 130℃; for 4h; Concentration; Time; | A 29% B 61% |

-
-
5122-99-6
4-iodophenylboronic acid

-
-
369-57-3
benzenediazonium tetrafluoroborate

-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With chloro[tris(para-trifluoromethylphenyl)phosphine]gold (I) In methanol at 20℃; Irradiation; Inert atmosphere; | 81% |

-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With chloroamine-T; sodium iodide In tetrahydrofuran; water at 20℃; for 0.333333h; | 80% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver nitrate In acetonitrile at 120℃; for 20h; Sealed tube; Schlenk technique; Inert atmosphere; | 80% |
-
-
18057-71-1
[1,1'-biphenyl-4-yl]triethylsilane

-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In acetonitrile at 20℃; for 24h; Inert atmosphere; | 79% |

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide at 80℃; for 3h; Catalytic behavior; Schlenk technique; Green chemistry; | 78% |
-
-
624-38-4
para-diiodobenzene

-
-
98-80-6
phenylboronic acid

-
A
-
92-94-4
[1,1';4',1'']terphenyl

-
B
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With potassium carbonate; copper-palladium In N,N-dimethyl-formamide at 110℃; for 24h; Suzuki cross-coupling; | A 75% B 25% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In 1,4-dioxane; methanol at 95℃; for 12h; Suzuki-Miyaura Coupling; Inert atmosphere; | A < 10 %Spectr. B 64% |


-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With potassium iodide In water at 20℃; | 75% |
-
-
624-38-4
para-diiodobenzene

-
-
71-43-2
benzene

-
A
-
92-94-4
[1,1';4',1'']terphenyl

-
B
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; N,N`-dimethylethylenediamine at 80℃; sealed tube; Inert atmosphere; | A 74% B 8% |
| With 1-(2-hydroxyethyl)piperazine; potassium tert-butylate at 100℃; for 24h; Sealed tube; Inert atmosphere; | A 69% B 7% |
| With potassium tert-butylate; vasicine at 20 - 110℃; for 48h; Schlenk technique; Inert atmosphere; | A 55% B 29% |
| With potassium tert-butylate for 6h; Inert atmosphere; Heating; High pressure; | A 21% B 30% |

-
-
91493-19-5
4-iodoxybiphenyl

-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| In nitrobenzene at 170℃; for 5h; | 72% |

| Conditions | Yield |
|---|---|
| With LiCrH4*2LiCl*2THF In tetrahydrofuran at 25℃; for 12h; | 100% |
| With potassium tert-butylate; benzyl alcohol In N,N-dimethyl-formamide at 90℃; for 2h; Reagent/catalyst; Solvent; Schlenk technique; Inert atmosphere; | 98% |
| With potassium tert-butylate; benzaldehyde In N,N-dimethyl-formamide at 90℃; for 2h; Schlenk technique; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 60℃; for 24h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With copper(l) chloride; potassium hydroxide at 50℃; for 3h; | 100% |
| With copper(l) chloride; potassium hydroxide at 50℃; for 3h; Ullmann Condensation; Inert atmosphere; | 100% |
-
-
64-17-5
ethanol

-
-
201230-82-2
carbon monoxide

-
-
1591-31-7
4-iodo-biphenyl

-
-
6301-56-0
ethyl 4-phenylbenzoate

| Conditions | Yield |
|---|---|
| With triethylamine; dichloro bis(acetonitrile) palladium(II) at 70℃; for 24h; | 99% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; [(Ph2CH=NOH)PdCl]2 In benzene at 120℃; for 3h; | 90% |


| Conditions | Yield |
|---|---|
| With caesium carbonate In water; N,N-dimethyl-formamide at 20℃; for 50h; Suzuki Coupling; Inert atmosphere; | 99% |
| With sodium acetate In water; N,N-dimethyl-formamide at 80℃; for 1h; Suzuki coupling; | 90% |
| With C33H48Cl2N4O9Pd; oxygen; potassium carbonate In water at 80℃; Suzuki-Miyaura Coupling; | 90% |
-
-
1591-31-7
4-iodo-biphenyl

-
-
19813-90-2
4-biphenylthiol

| Conditions | Yield |
|---|---|
| With copper(ll) sulfate pentahydrate; ethane-1,2-dithiol; potassium hydroxide In water; dimethyl sulfoxide at 90℃; for 20h; Inert atmosphere; | 99% |
| With sodiumsulfide nonahydrate; ethane-1,2-dithiol; copper(II) oxide In dimethyl sulfoxide at 120℃; for 20h; Inert atmosphere; Sealed tube; | 97% |
| With sodiumsulfide nonahydrate; copper; ethane-1,2-dithiol In dimethyl sulfoxide at 100℃; for 20h; Inert atmosphere; Green chemistry; | 97% |
| Stage #1: 4-iodo-biphenyl With copper(l) iodide; potassium carbonate; sulfur In N,N-dimethyl-formamide at 90℃; for 12h; Inert atmosphere; Stage #2: With sodium tetrahydroborate In N,N-dimethyl-formamide at 40℃; Inert atmosphere; Cooling with ice; | 84% |
| With copper(l) iodide; thiourea; L-proline; sodium t-butanolate In dimethyl sulfoxide at 90℃; for 24h; Inert atmosphere; | 83% |

| Conditions | Yield |
|---|---|
| With water; ethylene glycol; potassium hydroxide; copper dichloride In dimethyl sulfoxide at 120℃; for 24h; Reagent/catalyst; Temperature; Inert atmosphere; | 99% |
| Stage #1: 4-iodo-biphenyl With copper(l) iodide; cesiumhydroxide monohydrate; 1,3-diphenylpropanedione In water; dimethyl sulfoxide at 130℃; for 24h; Inert atmosphere; Stage #2: With hydrogenchloride In dichloromethane; water; dimethyl sulfoxide at 20℃; Inert atmosphere; chemoselective reaction; | 95% |
| Stage #1: 4-iodo-biphenyl With copper(l) iodide; 8-Hydroxyquinoline-N-oxide In dimethyl sulfoxide at 20℃; for 0.166667h; Inert atmosphere; Stage #2: With cesiumhydroxide monohydrate In water; dimethyl sulfoxide at 100℃; for 36h; Inert atmosphere; | 93% |
-
-
1591-31-7
4-iodo-biphenyl

-
-
87415-24-5
4-(perfluoropropyl)-1,1′-biphenyl

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 50℃; for 18h; Product distribution / selectivity; Inert atmosphere; | 99% |
-
-
1591-31-7
4-iodo-biphenyl

-
-
75264-92-5
tetramethylammonium trifluoromethylselenate(0)

-
-
1447204-44-5
4-[(trifluoromethyl)selanyl]-1,1′-biphenyl

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; bis(1,5-cyclooctadiene)nickel (0) In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; | 99% |
| With C54H76I2N4Ni2 In benzene at 45℃; Inert atmosphere; Glovebox; chemoselective reaction; | 99% |
| With palladium(l) tri-tert-butylphosphine iodide dimer In toluene at 40℃; for 24h; Glovebox; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With tri-tert-butylphosphonium tetrafluoroborate; N-Methyldicyclohexylamine; palladium diacetate In methanol; toluene at 120℃; under 11251.1 Torr; for 0.333333h; Wacker Oxidation; Flow reactor; | 99% |
| With N-Methyldicyclohexylamine; palladium diacetate; tri tert-butylphosphoniumtetrafluoroborate In methanol; toluene at 120℃; under 11251.1 Torr; Flow reactor; | 99% |
-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; manganese; (1,2-dimethoxyethane)dichloronickel(II) In N,N-dimethyl acetamide at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With copper In N,N-dimethyl-formamide at 60℃; for 12h; Schlenk technique; Inert atmosphere; Sealed tube; | 99% |
-
-
636-98-6
p-nitrobenzene iodide

-
-
1591-31-7
4-iodo-biphenyl

-
-
4612-26-4
1,4-Phenyldiboronic acid

-
-
25627-14-9
4-nitro-1,1':4',1'':4'',1'''-quaterphenyl

| Conditions | Yield |
|---|---|
| Stage #1: p-nitrobenzene iodide; 1,4-Phenyldiboronic acid With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In methanol; water; toluene Suzuki cross-coupling; Stage #2: 4-iodo-biphenyl With tetrakis(triphenylphosphine) palladium(0) In methanol; water; toluene for 32h; Reflux; | 98% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; triethylamine; triphenylphosphine In water at 60℃; for 24h; Sonogashira Cross-Coupling; Schlenk technique; Inert atmosphere; | 98% |
| With palladium diacetate; potassium carbonate In ethanol at 80℃; for 24h; | 88% |
| With copper(l) iodide; tetrakis(triphenylphosphine)palladium dichloride; triethylamine In tetrahydrofuran at 80℃; for 12h; Inert atmosphere; | 84% |

| Conditions | Yield |
|---|---|
| With (R)-1-(bis(4-methoxy-3,5-dimethylphenyl)phosphano)-2-((S)-1-(dicyclohexylphosphano)ethyl)ferrocene; palladium diacetate; thallium(I) acetate In toluene at 70℃; for 6h; optical yield given as %ee; enantioselective reaction; | 98% |
-
-
1591-31-7
4-iodo-biphenyl

-
-
106-54-7
p-Chlorothiophenol

-
-
1361124-61-9
biphenyl-4-yl(4-chlorophenyl)sulfane

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide In water at 50℃; for 24h; Inert atmosphere; Sealed tube; chemoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With 4.5Cu(2+)*4.5Ni(2+)*9C16H12N2*6C2O4(2-)*6H2O*P2W18O62(6-); sodium hydroxide In dimethyl sulfoxide at 120℃; for 12h; | 98% |
| With MOF-199; sodium hydroxide In dimethyl sulfoxide at 120℃; for 12h; Sealed tube; | 95% |
| With copper(ll) sulfate pentahydrate; sodium hydroxide In dimethyl sulfoxide at 110℃; for 12h; Sealed tube; | 86% |
| With copper(II) acetate monohydrate; caesium carbonate In N,N-dimethyl-formamide at 20 - 110℃; for 24h; Inert atmosphere; Schlenk technique; | 82% |
-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
1591-31-7
4-iodo-biphenyl

-
-
2920-38-9
4-cyano-1,1'-biphenyl

| Conditions | Yield |
|---|---|
| With copper (II) trifluoroacetate hydrate; palladium diacetate In dimethyl sulfoxide at 130℃; for 12h; Reagent/catalyst; Solvent; Sealed tube; Inert atmosphere; | 98% |
-
-
1591-31-7
4-iodo-biphenyl

| Conditions | Yield |
|---|---|
| With bis[2-(diphenylphosphino)phenyl] ether; bis(dibenzylideneacetone)-palladium(0) In toluene at 50℃; for 12h; | 98% |
-
-
1663-39-4
tert-Butyl acrylate

-
-
1591-31-7
4-iodo-biphenyl

-
-
910574-67-3
tert-butyl(E)-3-([1,1′-biphenyl]-4-yl)acrylate

| Conditions | Yield |
|---|---|
| With di-μ-iodobis(tri-t-butylphosphino)dipalladium(l); N-ethyl-N,N-diisopropylamine In toluene at 100℃; for 15h; Heck Reaction; Inert atmosphere; Sealed tube; Glovebox; | 98% |
-
-
401-55-8
ethyl bromofluoroacetate

-
-
1591-31-7
4-iodo-biphenyl

-
-
1234908-38-3
ethyl 2-(biphenyl-4-yl)-2-fluoroacetate

| Conditions | Yield |
|---|---|
| With bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; tetrabutylammomium bromide; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; zinc In tetrahydrofuran at 60℃; for 24h; Inert atmosphere; | 98% |
-
-
1591-31-7
4-iodo-biphenyl

-
-
1066-54-2
trimethylsilylacetylene

-
-
75867-42-4
([1,1'-biphenyl]-4-ylethynyl)trimethylsilane

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 20℃; for 1h; | 97% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide In triethylamine for 4h; Ambient temperature; | 86% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine In acetonitrile at 20℃; for 24h; Sonogashira Cross-Coupling; Schlenk technique; Inert atmosphere; | 86% |
-
-
1591-31-7
4-iodo-biphenyl

-
-
177551-63-2
[1,1′-biphenyl]-4-yl(trifluoromethyl)sulfane

| Conditions | Yield |
|---|---|
| With palladium(l) tri-tert-butylphosphine iodide dimer In toluene at 80℃; | 97% |
| With bis(1,5-cyclooctadiene)nickel(0); 4,4'-Dimethoxy-2,2'-bipyridin In tetrahydrofuran at 20℃; for 22h; Inert atmosphere; | 92% |
-
-
95-16-9
1,3-Benzothiazole

-
-
1591-31-7
4-iodo-biphenyl

-
-
403517-20-4
2-([1,1'-biphenyl]-4-ylthio)benzenamine

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide; water; copper(l) chloride at 50℃; for 12h; Inert atmosphere; | 97% |
4-Iodobiphenyl Chemical Properties
IUPAC Name:1-Iodo-4-phenylbenzene
Molecular Formula: C12H9I
Molecular Weight: 280.104330 g/mol
EINECS:216-469-1
Melting Point: 110-114 °C
Density: 1.584 g/cm3
Flash Point: 145.8 °C
Sensitive: Light
Boiling Point: 322.7 °C at 760 mmHg
Vapour Pressure: 0.000517 mmHg at 25 °C
Water Solubility: 0.9974 mg/L at 25 °C
Molecular Structure of 4-Iodobiphenyl (1591-31-7):
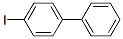
Categories of 4-Iodobiphenyl (1591-31-7): Biphenyl derivatives;pharmacetical;Halides;Phenyls & Phenyl-Het;Halogenated Hydrocarbons
4-Iodobiphenyl Uses
4-Iodobiphenyl (1591-31-7) is used as an intermediate of pharmaceutical and LCD.
4-Iodobiphenyl Safety Profile
Hazard Codes: Xn,
Xn, Xi,
Xi, N
N
Risk Statements:22-41-50/53-36/37/38
22:Harmful if swallowed
41:Risk of serious damage to eyes
50/53:Very Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements:26-39-60-61-37/39
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
39:Wear eye/face protection
60:This material and/or its container must be disposed of as hazardous waste
61:Avoid release to the environment. Refer to special instructions safety data sheet
37/39:Wear suitable gloves and eye/face protection
4-Iodobiphenyl Specification
First Aid Measures of 4-Iodobiphenyl (1591-31-7):
Ingestion:If swallowed, wash out mouth with water provided person is conscious. Call a physician.
Inhalation:If inhaled, remove to fresh air. If not breathing give artificial respiration. If breathing is difficult, give oxygen.
Skin:In case of skin contact, flush with copious amounts of water for at least 15 minutes. Remove contaminated clothing and shoes. Call a physician.
Eyes:In case of contact with eyes, flush with copious amounts of water for at least 15 minutes. Assure adequate flushing by separating the eyelids with fingers. Call a physician.
Related Products
- 4-Iodobiphenyl
- 159138-80-4
- 15914-84-8
- 1591-56-6
- 15917-09-6
- 159171-78-5
- 159175-58-3
- 15917-77-8
- 159177-91-0
- 159178-03-7
- 159191-56-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View