-
Name
6-Methyl-5-hepten-2-one
- EINECS 203-816-7
- CAS No. 110-93-0
- Article Data192
- CAS DataBase
- Density 0.835 g/cm3
- Solubility insoluble in water
- Melting Point -67.1 ºC
- Formula C8H14O
- Boiling Point 173.3 ºC at 760 mmHg
- Molecular Weight 126.199
- Flash Point 50.6 ºC
- Transport Information UN 1224 3/PG 3
- Appearance Clear slightly yellow liquid
- Safety 16
- Risk Codes 10
-
Molecular Structure
- Hazard Symbols R10:;
- Synonyms 2-Methyl-2-hepten-6-one;2-Methyl-2-heptene-6-ketone;2-Methyl-2-heptene-6-one;2-Methyl-6-oxo-2-heptene;2-Oxo-6-methylhept-5-ene;6-Methyl-5-hepten-2-ketone;6-Methyl-D5-hepten-2-one;Methylheptenone;NSC 15294;NSC 66569;Prenylacetone;Sulcatone;
- PSA 17.07000
- LogP 2.32180
Synthetic route
-
-
29171-20-8
3,7-dimethyloct-6-en-1-yn-3-ol

-
-
115-19-5
2-methyl-but-3-yn-2-ol

-
A
-
110-93-0
6-Methyl-hept-5-en-2-on

-
B
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| potassium hydroxide In water at 93℃; for 6.41667h; Heating / reflux; | A 95.2% B 95.8% |


| Conditions | Yield |
|---|---|
| With Dimethyl phosphite at 150℃; for 14.6h; Reagent/catalyst; Time; Inert atmosphere; | 95.1% |
| hydrogen tris(oxalato)phosphate In methanol at 124.84 - 149.84℃; for 17 - 25h; Product distribution / selectivity; | 77.3% |
| hydrogen tris(oxalato)phosphate In acetone at 149.84℃; for 24h; Product distribution / selectivity; | 56.4% |
-
-
74596-83-1
6-methylhept-5-en-2-one N,N-dimethylhydrazone

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With sodium perborate; sodium hydroxide; potassium dihydrogenphosphate; water In tert-butyl alcohol at 60℃; for 24h; | 94% |
-
-
2190-48-9
3-chloro-3-methyl-1-butene

-
-
503-60-6
3,3-dimethyl-allyl chloride

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide; sodium hydroxide In acetone at 50℃; for 2h; Reagent/catalyst; Temperature; Inert atmosphere; | 92.8% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; potassium permanganate; neutral In acetone for 4h; Ambient temperature; | A n/a B 92% |
-
-
5392-40-5
(E/Z)-3,7-dimethyl-2,6-octadienal

-
A
-
110-93-0
6-Methyl-hept-5-en-2-on

-
B
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide; water at 150℃; for 0.166667h; Microwave irradiation; Green chemistry; | A 91% B n/a |
| With potassium carbonate |
-
-
115-18-4
2-methyl-3-buten-2-ol

-
-
105-45-3
acetoacetic acid methyl ester

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| 90.8% |

| Conditions | Yield |
|---|---|
| With pyrrolidine; water In acetonitrile at 20℃; for 12h; | 90% |
| With water; potassium carbonate man destilliert mit Wasserdampf und fraktioniert im Vakuum; | |
| With potassium carbonate |

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide; dihydrogen peroxide In methanol at 20℃; pH=7; aq. phosphate buffer; | 89% |
-
-
95-54-5
1,2-diamino-benzene

-
-
5392-40-5
(E/Z)-3,7-dimethyl-2,6-octadienal

-
A
-
615-15-6
2-Methyl-1H-benzimidazole

-
B
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydroxide; water at 150℃; for 0.166667h; Microwave irradiation; Green chemistry; | A 61% B 89% |


| Conditions | Yield |
|---|---|
| With polymer-supported dicyanoketene acetal; water at 20℃; for 20h; | 88% |
| With silica gel; toluene-4-sulfonic acid In dichloromethane for 3h; | 87% |
| With polymer-supported dicyanoketene acetal; water In acetonitrile at 20℃; for 20h; Hydrolysis; | 73% |
| palladium (II) ion In acetone for 1h; Ambient temperature; | 100 % Chromat. |

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| Stage #1: C10H17NO2 With chromium dichloride; acrylic acid methyl ester In tetrahydrofuran at 25℃; Stage #2: With water In tetrahydrofuran Further stages.; | 88% |
-
-
116-11-0
2-Methoxypropene

-
-
1196448-62-0
3-(1-methoxy-1-methyl-ethoxy)-3-methyl-but-1-ene

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| Stage #1: 3-(1-methoxy-1-methyl-ethoxy)-3-methyl-but-1-ene at 150℃; for 16h; autoclave; Stage #2: 2-Methoxypropene; phosphoric acid In acetone at 150℃; for 16h; autoclave; | 86% |
-
-
4630-06-2, 1569-60-4
6-methylhept-5-en-2-ol

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With trimethylsilyl chromates In dichloromethane at 25℃; for 2h; | 85% |
| With butyltriphenylphosphonium dichromate In chloroform for 3.5h; Oxidation; Heating; | 80% |
| With [Fe[(S)-N-benzyl-2-phenyl-2-(pyridin-2-ylmethoxy)-N-(pyridin-2-ylmethyl)ethanamine](OTf)2]; dihydrogen peroxide In water; acetonitrile at 20℃; for 2h; Inert atmosphere; | 69% |
-
-
80868-07-1
6-Methyl-3-(toluene-4-sulfonyl)-hept-5-en-2-one

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate; sodium amalgam In methanol at 0℃; for 2h; | 85% |
-
-
140458-17-9
4-<(tert-Butyl)dimethylsilyl>-2-methyl-2-(4'-methylpent-3'-enyl)-1,3-dioxole

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With water; oxalic acid Ambient temperature; | 85% |
-
-
15973-38-3
1,1-dimethylprop-2-enyl 3-oxobutanoate

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With aluminum oxide at 60℃; for 3h; | 84% |
| With aluminum oxide at 60℃; for 3h; Product distribution; substrate supported on Al2O3; Carrol rearrangement of allyl acetoacetates to γ,δ-unsaturated ketones on the surface of chromatographic absorbents; | 84% |
| With aluminum isopropoxide at 160℃; |
-
-
29171-20-8
3,7-dimethyloct-6-en-1-yn-3-ol

-
-
75-66-1
2-methylpropan-2-thiol

-
A
-
110-93-0
6-Methyl-hept-5-en-2-on

-
B
-
86254-75-3
(Z)-1-tert-Butylsulfanyl-3,7-dimethyl-octa-1,6-dien-3-ol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 50℃; for 6h; | A 10% B 83% |
| With potassium hydroxide In dimethyl sulfoxide at 50℃; for 6h; | A 10% B 83% |

-
-
22418-73-1
6-methylhept-5-en-2-one oxime

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; dipyridinium dichromate In dichloromethane at 0℃; for 4.5h; | 80% |
-
-
140458-18-0
2,4-Dimethyl-2-(4'-methylpent-3'-enyl)-1,3-dioxole

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 24h; | 80% |
-
-
5392-40-5
(E/Z)-3,7-dimethyl-2,6-octadienal

-
A
-
110-93-0
6-Methyl-hept-5-en-2-on

-
B
-
61447-89-0, 474779-14-1
4,6-bis(4-methylpent-3-en-1-yl)-6-methylcyclohexa-1,3-diene-1-carbaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydride In diethyl ether for 0.5h; Heating; | A n/a B 80% |
-
-
4630-06-2, 1569-60-4
6-methylhept-5-en-2-ol

-
A
-
110-93-0
6-Methyl-hept-5-en-2-on

-
B
-
10408-15-8
6-methylhept-6-en-2-one

-
C
-
928-68-7
6-methylheptan-2-one

| Conditions | Yield |
|---|---|
| With reusable unsupported rhenium nanocrystalline particle In neat (no solvent) at 180℃; for 10h; Green chemistry; | A 80% B 5% C 15% |

-
-
1196448-62-0
3-(1-methoxy-1-methyl-ethoxy)-3-methyl-but-1-ene

-
-
77-76-9
2,2-dimethoxy-propane

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| phosphoric acid In acetone at 150℃; for 32h; Product distribution / selectivity; autoclave; | 78.8% |
-
-
16996-12-6
2,3-epoxygeranial

-
A
-
50340-32-4
2-methyl-2-(4-methyl-pent-3-enyl)-oxirane

-
B
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In 1,2-dichloro-benzene at 140℃; for 14h; Sealed tube; Inert atmosphere; | A 73% B 23% |
-
-
1694-31-1
tert-butyl acetoacetate

-
-
115-18-4
2-methyl-3-buten-2-ol

-
A
-
110-93-0
6-Methyl-hept-5-en-2-on

-
B
-
15973-38-3
1,1-dimethylprop-2-enyl 3-oxobutanoate

| Conditions | Yield |
|---|---|
| In toluene for 4h; Heating; | A 31% B 72% |

-
-
15973-38-3
1,1-dimethylprop-2-enyl 3-oxobutanoate

-
A
-
110-93-0
6-Methyl-hept-5-en-2-on

-
B
-
21597-32-0
3-methylbut-2-en-1-yl 3-oxobutanoate

| Conditions | Yield |
|---|---|
| With silica gel at 60℃; for 5h; | A n/a B 70% |

| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; for 3h; | 70% |
| In diethyl ether at 0℃; for 1h; Methylation; | 70% |
-
-
29171-20-8
3,7-dimethyloct-6-en-1-yn-3-ol

-
-
86254-77-5
3-tert-Butylsulfanyl-but-3-en-2-ol

-
A
-
110-93-0
6-Methyl-hept-5-en-2-on

-
B
-
86254-75-3
(Z)-1-tert-Butylsulfanyl-3,7-dimethyl-octa-1,6-dien-3-ol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In neat (no solvent) at 75 - 120℃; for 0.366667h; | A 33% B 64% |

-
-
115-18-4
2-methyl-3-buten-2-ol

-
-
141-97-9
ethyl acetoacetate

-
A
-
110-93-0
6-Methyl-hept-5-en-2-on

-
B
-
21597-32-0
3-methylbut-2-en-1-yl 3-oxobutanoate

| Conditions | Yield |
|---|---|
| In paraffin 1.) 160 deg C, 6 h, 2.) 180 deg C, 3 h; | A 60% B 5% |

-
-
1196448-62-0
3-(1-methoxy-1-methyl-ethoxy)-3-methyl-but-1-ene

-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| Stage #1: 3-(1-methoxy-1-methyl-ethoxy)-3-methyl-but-1-ene at 150℃; for 16h; autoclave; Stage #2: phosphoric acid In acetone at 150℃; for 16h; autoclave; | 60% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
4630-06-2, 1569-60-4
6-methylhept-5-en-2-ol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether at 0℃; for 1h; | 100% |
| With lithium aluminium tetrahydride In diethyl ether at 0℃; for 1h; | 99% |
| With hydrogen; silver trifluoromethanesulfonate; potassium hexamethylsilazane In toluene at 25℃; under 15001.5 Torr; for 24h; Glovebox; chemoselective reaction; | 99% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
58917-26-3
(S)-sulcatol

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 50℃; for 24h; ADH T. brockii (growing), NADP, PAN 800; | 100% |
| With NADH-dependent-formate-dehydrogenase; flavin adenine dinucleotide; NADH at 30℃; for 6h; pH=7.5; aq. buffer; Enzymatic reaction; optical yield given as %ee; enantiospecific reaction; | 99% |
| With dichloro(benzene)ruthenium(II) dimer; mono(3-deoxy-3-[N-(2-hydroxyethyl)amino])-β-cyclodextrin; sodium formate In water; N,N-dimethyl-formamide at 50℃; for 12h; optical yield given as %ee; enantioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 0 - 5℃; for 0.5h; Inert atmosphere; | 99.1% |
| 86% | |
| With diethyl ether unter Zusatz von Kaliumhydroxid; |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate under 2280 Torr; for 0.75h; Ambient temperature; | 99% |
| With hydrogen In tetrahydrofuran at 24.84℃; under 15001.5 Torr; for 1h; | 98.2% |
| With hydrogen; palladium/alumina at 60℃; under 1500.15 Torr; for 66h; Product distribution / selectivity; Autoclave; | 97.3% |

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate at 20℃; for 4h; | 99% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
77086-38-5
ketene t-butyldimethylsilyl methyl acetal

| Conditions | Yield |
|---|---|
| Stage #1: 6-Methyl-hept-5-en-2-on With bis(trifluoromethanesulfonyl)amide In diethyl ether at -78 - 23℃; Mukaiyama Aldol Addition; Schlenk technique; Inert atmosphere; Stage #2: ketene t-butyldimethylsilyl methyl acetal In diethyl ether at -20℃; for 0.5h; Mukaiyama Aldol Addition; Schlenk technique; Inert atmosphere; | 99% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
2181-44-4
trimethylsulfonium methylsulfate

-
-
50340-32-4
2-methyl-2-(4-methyl-pent-3-enyl)-oxirane

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride In dichloromethane | 98% |

| Conditions | Yield |
|---|---|
| With formic acid; chloro-(pentamethylcyclopentadienyl)-{5-methoxy-2-{1-[(4-methoxyphenyl)imino-N]ethyl}phenyl-C}-iridium(lll); triethylamine In methanol at 80℃; for 1h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: Vinyl bromide With n-butyllithium; lithium bromide In tetrahydrofuran; diethyl ether; pentane at -127 - -105℃; for 3h; Inert atmosphere; Schlenk technique; Stage #2: 6-Methyl-hept-5-en-2-on In tetrahydrofuran; diethyl ether; pentane Inert atmosphere; Schlenk technique; | 97% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
3536-96-7
vinylmagnesium chloride

-
-
78-70-6
3,7-dimethylocta-1,6-dien-3-ol

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In tetrahydrofuran at 20℃; for 4h; Reagent/catalyst; Solvent; Inert atmosphere; Cooling with ice; | 96.3% |
| With tetrahydrofuran |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
107-21-1
ethylene glycol

-
-
3695-38-3
2-methyl-2-(4-methyl-3-pentenyl)-1,3-dioxolane

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In benzene Heating; | 96% |
| With toluene-4-sulfonic acid In benzene | 94% |
| With toluene-4-sulfonic acid In benzene for 5h; Condensation; Heating; | 92% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
624-65-7
2-propynyl chloride

-
-
71869-02-8
4,8-dimethyl-non-7-en-1-yn-4-ol

| Conditions | Yield |
|---|---|
| With manganese; TiCpCl2 In tetrahydrofuran at 20℃; for 1h; Reagent/catalyst; Inert atmosphere; | 96% |
| With 2,4,6-trimethyl-pyridine; bis(cyclopentadienyl)titanium dichloride; manganese; chloro-trimethyl-silane In tetrahydrofuran at 20℃; for 7h; Barbier Coupling Reaction; Inert atmosphere; chemoselective reaction; | 96% |
-
-
1271-66-5
dimethyltitanocene

-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
6709-39-3
2,6-dimethyl-hepta-1,5-diene

| Conditions | Yield |
|---|---|
| Stage #1: dimethyltitanocene; 6-Methyl-hept-5-en-2-on In toluene at 20 - 80℃; for 5h; Inert atmosphere; Stage #2: With sodium hydrogencarbonate In methanol; water; toluene at 40℃; for 3h; Temperature; | 95.3% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
1826-67-1
vinyl magnesium bromide

-
-
78-70-6
3,7-dimethylocta-1,6-dien-3-ol

| Conditions | Yield |
|---|---|
| With zinc(II) chloride In tetrahydrofuran at 20℃; for 5h; Reagent/catalyst; Solvent; Inert atmosphere; Cooling with ice; | 95.2% |
| With tetrahydrofuran; diethyl ether | |
| In tetrahydrofuran at -78℃; Addition; |
-
-
917-64-6
methyl magnesium iodide

-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
6090-15-9
2,6-dimethylhept-5-en-2-ol

| Conditions | Yield |
|---|---|
| In diethyl ether for 1h; | 95% |
| analog verlaeuft die Reaktion mit Magnesiumaethyljodid; |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
105-36-2
ethyl bromoacetate

-
-
54211-39-1
3-hydroxy-3,7-dimethyl-oct-6-enoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With zinc In tetrahydrofuran for 2h; Reformatsky Reaction; Reflux; | 95% |
| With zinc In tetrahydrofuran for 2h; Reformatsky Reaction; Reflux; | 95% |
| With zinc In tetrahydrofuran for 2h; Reformatsky Reaction; Reflux; | 95% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
84569-39-1
6-methylhept-5-en-2-yl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| With tert-Amyl alcohol; sodium hydride; zinc(II) chloride In tetrahydrofuran for 0.25h; Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; | 95% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
4301-14-8
acetylenemagnesium bromide

-
-
29171-20-8
3,7-dimethyloct-6-en-1-yn-3-ol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 1h; | 95% |
| In tetrahydrofuran; diethyl ether Grignard reaction; | 90% |
| In tetrahydrofuran at -78 - 20℃; | 87% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
54211-39-1
3-hydroxy-3,7-dimethyl-oct-6-enoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With zinc Reformatsky reaction; | 95% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With sulfuric acid; dihydrogen peroxide; trifluoroacetic acid In dichloromethane at 0℃; for 6h; | 95% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate at 0 - 35℃; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 6-Methyl-hept-5-en-2-on With (R,R)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminoaluminum(III) chloride; (tert-Butoxycarbonylmethylene)triphenylphosphorane; Triphenylphosphine oxide In dichloromethane at -30℃; for 0.5h; Inert atmosphere; Stage #2: trimethylsilyl cyanide In dichloromethane at -30℃; for 20h; Inert atmosphere; | 95% |
| With (R,R)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediaminoaluminum(III) chloride; ethyl (triphenylphosphoranylidene)acetate In ethyl acetate at -30℃; for 33h; Solvent; Inert atmosphere; | 92% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
16194-30-2
5,6-dihydroxy-6-methyl-heptan-2-one

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide; 4-methylmorpholine N-oxide In water; acetone; tert-butyl alcohol at 0℃; for 4h; | 94% |
| With water; oxygen bei der Sonnenbelichtung; | |
| With potassium permanganate; water | |
| With potassium permanganate |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
17745-52-7
N,N-Bis(trimethylsilyl)benzenesulphenamide

-
-
130485-93-7
N-<(6-methyl)-5-hepten-2-ylidene>benzenesulfenamide

| Conditions | Yield |
|---|---|
| tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; | 94% |
-
-
110-93-0
6-Methyl-hept-5-en-2-on

-
-
57-14-7
1,1-dimethylhydrazine

-
-
74596-83-1
6-methylhept-5-en-2-one N,N-dimethylhydrazone

| Conditions | Yield |
|---|---|
| With acetic acid at 20℃; for 3h; Condensation; | 94% |
| Yield given; |

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl methane phosphonate With n-butyllithium In tetrahydrofuran; hexane at -70℃; for 0.25h; Stage #2: 6-Methyl-hept-5-en-2-on In tetrahydrofuran; hexane for 0.0833333h; Further stages.; | 94% |
6-Methyl-5-hepten-2-one Consensus Reports
Reported in EPA TSCA Inventory.
6-Methyl-5-hepten-2-one Specification
The 6-Methyl-5-hepten-2-one, with the CAS registry number 110-93-0, is also known as Methylheptenone. It belongs to the product categories of Alphabetical Listings; Certified Natural Products Flavors and Fragrances; Flavors and Fragrances; M-N; 7 to C8; Carbonyl Compounds; Ketones. Its EINECS registry number is 203-816-7. This chemical's molecular formula is C8H14O and molecular weight is 126.2. What's more, its IUPAC name is the same with its product name. When you are dealing with this chemical, you should be very careful. This chemical is flammable. So you should keep away from sources of ignition.
Physical properties about 6-Methyl-5-hepten-2-one are: (1)ACD/LogP: 2.09; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.09; (4)ACD/LogD (pH 7.4): 2.09; (5)ACD/BCF (pH 5.5): 23; (6)ACD/BCF (pH 7.4): 23; (7)ACD/KOC (pH 5.5): 328.34; (8)ACD/KOC (pH 7.4): 328.34; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 17.07 Å2; (13)Index of Refraction: 1.43; (14)Molar Refractivity: 39.04 cm3; (15)Molar Volume: 151.1 cm3; (16)Surface Tension: 25.2 dyne/cm; (17)Density: 0.835 g/cm3; (18)Flash Point: 50.6 °C; (19)Enthalpy of Vaporization: 40.96 kJ/mol; (20)Boiling Point: 173.3 °C at 760 mmHg; (21)Vapour Pressure: 1.28 mmHg at 25 °C.
Preparation of 6-Methyl-5-hepten-2-one: this chemical can be prepared by Acetoacetic acid-(1,1-dimethyl-allyl ester). This reaction needs reagent aluminium isopropylate at temperature of 160 °C.
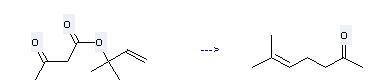
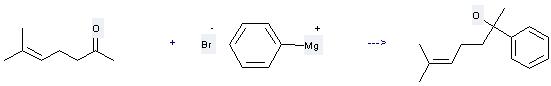
Uses of 6-Methyl-5-hepten-2-one: (1) it is used as the raw material of synthetic spices and used in baked goods, meat, sugar, beverage; (2) it is used to produce other chemicals. For example, it can react with Phenylmagnesium bromide to get 6-Methyl-2-phenyl-hept-5-en-2-ol. The reaction occurs with reagent diethyl ether at ambient temperature. The yield is 60 %.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C(C)CC\C=C(/C)C
(2) InChI: InChI=1S/C8H14O/c1-7(2)5-4-6-8(3)9/h5H,4,6H2,1-3H3
(3) InChIKey: UHEPJGULSIKKTP-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 2410mg/kg (2410mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 32(8), Pg. 52, 1988. |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 859, 1975. | |
| rat | LD50 | oral | 3500mg/kg (3500mg/kg) | Medizin und Ernaehrung. Vol. 8, Pg. 244, 1967. | |
| rat | LD50 | skin | > 2gm/kg (2000mg/kg) | Food and Cosmetics Toxicology. Vol. 13, Pg. 859, 1975. |
Related Products
- 6-Methyl-5-hepten-2-one
- 110935-21-2
- 11093-65-5
- 110-94-1
- 110942-02-4
- 110945-00-1
- 11094-60-3
- 110-95-2
- 110960-73-1
- 11096-26-7
- 110-96-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View