-
Name
Acetovanillone
- EINECS 207-854-5
- CAS No. 498-02-2
- Article Data87
- CAS DataBase
- Density 1.158 g/cm3
- Solubility soluble in hot water
- Melting Point 112-115 °C(lit.)
- Formula C9H10O3
- Boiling Point 297.5 °C at 760 mmHg
- Molecular Weight 166.177
- Flash Point 125.5 °C
- Transport Information
- Appearance yellowish to brown powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Acetophenone,4'-hydroxy-3'-methoxy- (8CI);1-(4-Hydroxy-3-methoxyphenyl)ethanone;2-Methoxy-4-acetylphenol;3'-Methoxy-4'-hydroxyacetophenone;4-Acetyl-2-methoxyphenol;4-Acetylguaiacol;4-Hydroxy-3-methoxyphenyl methylketone;4'-Hydroxy-3'-methoxyacetophenone;Acetoguaiacon;Acetoguaiacone;Acetoguaicone;Apocynin;Apocynine;
- PSA 46.53000
- LogP 1.60340
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxy-4-hydroxybenzoic acid; 4CH3(1-)*Zn(2+)*Li(1+) In tetrahydrofuran; diethyl ether; hexane at 20℃; Schlenk technique; Inert atmosphere; Stage #2: With iodine In tetrahydrofuran; diethyl ether; hexane at 20℃; Solvent; Schlenk technique; Inert atmosphere; chemoselective reaction; | 96% |
-
-
7382-68-5
1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)ethanol

-
A
-
90-05-1
2-methoxy-phenol

-
B
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; palladium on activated charcoal In water; ethyl acetate at 80℃; for 14h; | A n/a B 96% |
| With C32H25Cl2N6O2Rh2(1+)*Cl(1-); sodium hydroxide In water at 110℃; for 18h; Inert atmosphere; | A 86% B 82% |
| With C32H25Cl2N6O2Rh2(1+)*Cl(1-); sodium hydroxide In water at 110℃; for 18h; Inert atmosphere; Green chemistry; | A 86% B 82% |
| With C22H17Cl3N3ORh; sodium hydroxide In water for 60h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In nitrobenzene at 40℃; for 3h; Reagent/catalyst; Temperature; Large scale; | 90.23% |
| With methanesulfonic acid In dichloromethane under 15751.6 Torr; Reagent/catalyst; Fries Phenol Ester Rearrangement; Flow reactor; | 75% |
| With methanesulfonic acid; phosphorus pentoxide at 90℃; for 5h; Fries rearrangement; | 67% |
-
-
22317-35-7
1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)ethan-1-one

-
A
-
90-05-1
2-methoxy-phenol

-
B
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With formic acid; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 24h; Inert atmosphere; Irradiation; | A 84% B 78% |
| With RuH2(CO)(PPh3)3; hydrogen; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In (2)H8-toluene at 135℃; under 760.051 Torr; for 20h; |
-
-
1197-09-7
1-(3,4-dihydroxyphenyl)ethan-1-one

-
-
74-88-4
methyl iodide

-
A
-
6100-74-9
3-hydroxy-4-methoxyacetophenone

-
B
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
C
-
1131-62-0
1-(3,4-dimethoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With lithium carbonate In N,N-dimethyl-formamide at 55℃; for 18h; Inert atmosphere; chemoselective reaction; | A 84% B 6% C 4% |


| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol at 80℃; for 1h; | A 80% B 5% C 83% |


| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate In carbon disulfide for 6h; Friedel-Crafts reaction; Heating; | 79% |
-
-
1445875-26-2
C18H30O3Si

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With water; acetic acid; N,N,N',N'-tetramethylguanidine In tetrahydrofuran; acetonitrile at 20℃; | 75% |
| With acetic acid In tetrahydrofuran; water; acetonitrile at 20℃; for 12h; pH=7.5; | 63% |
-
-
84272-48-0
4-hydroxy-3-methoxybenzoylacetic acid

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sulfuric acid Heating; | 66% |
-
-
132255-78-8
4'-tert-butyldimethylsilyloxy-3'-methoxyacetophenone

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With acetic acid In tetrahydrofuran; water; acetonitrile at 20℃; for 12h; pH=7.5; | 63% |
| With water; acetic acid; N,N,N',N'-tetramethylguanidine In tetrahydrofuran; acetonitrile at 20℃; | 63% |

| Conditions | Yield |
|---|---|
| With poly phosphoric acid (PPA) at 100℃; for 0.333333h; Time; | 44.2% |
-
-
7382-68-5
1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)ethanol

-
A
-
2480-86-6
Apocynol

-
B
-
90-05-1
2-methoxy-phenol

-
C
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
D
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; nickel at 25℃; Product distribution; Further Variations:; Temperatures; other substrates, other current densities; Catalytic hydrogenation; Electrochemical reaction; | A 41% B 43% C 1.3% D 2% |


| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; copper(l) chloride In acetonitrile at 25℃; under 735.576 Torr; for 20h; chemoselective reaction; | A 35% B 12% C 9% |

-
-
906668-05-1
2-(2,6-dimethoxyphenoxy)-1-(4-hydroxy-3-methoxyphenyl)ethan-1-one

-
A
-
91-10-1
1,3-dimethoxy-2-hydroxy-benzene

-
B
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With lithium tetrafluoroborate In N,N-dimethyl-formamide at 20℃; for 2.5h; Electrolysis; | A 28% B 22% |
-
-
2480-86-6
Apocynol

-
A
-
2880-58-2
2-methoxy-1,4-benzoquinone

-
B
-
121-33-5
vanillin

-
C
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetonitrile for 0.5h; Microwave irradiation; | A 23% B 5% C 18% |

-
-
1135-24-6
(E)-3-(4-hydroxy-3-methoxyphenyl)acrylic acid

-
A
-
3943-74-6
4-hydroxy-3-methoxybenzoic acid methyl ester

-
B
-
121-33-5
vanillin

-
C
-
84272-48-0
4-hydroxy-3-methoxybenzoylacetic acid

-
D
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With salcomine; oxygen In methanol at 25℃; under 7600 Torr; for 72h; | A 20% B 16% C n/a D n/a |
| With salcomine; oxygen In methanol at 25℃; under 7600 Torr; for 72h; Product distribution; also with following CH2N2/ether action, other products; | A 20% B 16% C n/a D n/a |


| Conditions | Yield |
|---|---|
| With prolinium tetrachloromanganate(II); ethylammonium nitrate (EAN) at 35℃; for 4h; Time; | A 20% B n/a |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetonitrile for 0.5h; Reagent/catalyst; Microwave irradiation; | 18% |
| With 2,3-dicyano-5,6-dichloro-p-benzoquinone In benzene for 4h; | |
| With sodium phosphate buffer; DH-I and DH-II of Pseudomonas sp. TMY 1009; NAD; acetaldehyde; yeast alcohol dehydrogenase In water |

| Conditions | Yield |
|---|---|
| With prolinium tetrachloromanganate(II); ethylammonium nitrate (EAN) at 35℃; for 6h; | A n/a B 12% C n/a |

-
-
2480-86-6
Apocynol

-
A
-
2880-58-2
2-methoxy-1,4-benzoquinone

-
B
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetonitrile for 0.5h; Microwave irradiation; | A 11% B 10% |
| With dihydrogen peroxide In water; acetonitrile at 39.84℃; for 0.25h; Reagent/catalyst; | A 43 %Chromat. B 52 %Chromat. |
| With dihydrogen peroxide In water; acetonitrile at 39.84℃; for 0.25h; Reagent/catalyst; Time; | A 65 %Chromat. B 19 %Chromat. |

-
A
-
104-76-7
2-Ethylhexyl alcohol

-
B
-
121-33-5
vanillin

-
C
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methyl imidazolium tetrachloromanganate(II); ethylammonium nitrate (EAN) at 35℃; for 6h; | A n/a B 10% C n/a |

-
-
917-64-6
methyl magnesium iodide

-
-
790-16-9
4-benzoyloxy-3-methoxybenzaldehyde

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| durch Ueberfuehrung in das Carbinol, Oxydation mit Kaliumdichromat und Schwefelsaeure zu 3-Methoxy-4-benzoyloxy-acetophenon und Verseifen durch Kochen mit verd.Natronlauge; |
-
-
6344-28-1
2-chloro-1-(4-hydroxy-3-methoxyphenyl)ethan-1-one

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sulfuric acid; iron; sodium iodide |

| Conditions | Yield |
|---|---|
| With zinc(II) chloride | |
| With aluminium trichloride; zinc(II) chloride at 140 - 150℃; | |
| With boron trifluoride | |
| With PPA |
-
-
1131-62-0
1-(3,4-dimethoxyphenyl)ethanone

-
A
-
6100-74-9
3-hydroxy-4-methoxyacetophenone

-
B
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With hydrogen bromide for 3h; Heating; | A 0.2 g B 0.05 g |
| Stage #1: 1-(3,4-dimethoxyphenyl)ethanone With aluminum (III) chloride; thiourea In dichloromethane for 5h; Heating / reflux; Stage #2: With hydrogenchloride In dichloromethane; water at 20℃; |
-
-
7382-59-4
guaiacylglycerol-β-guaiacyl ether

-
A
-
2196-18-1
3-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-1-propanone

-
B
-
90-05-1
2-methoxy-phenol

-
C
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With oxygen; rose bengal In acetonitrile at 13℃; Irradiation; | A 28 % Chromat. B 15 % Chromat. C 30 % Chromat. |

-
-
143418-77-3
1-<3-methoxy-4-(methoxymethoxy)phenyl>ethanone

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| hydrogenchloride In methanol Yield given; |
-
-
54771-60-7
4-acetyl-2-methoxyphenyl acetate

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With piperidine In 1,4-dioxane at 25℃; Kinetics; Deacetylation; |

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sulfuric acid; iron at 60℃; |
-
-
100-44-7
benzyl chloride

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
1835-11-6
4-benzyloxy-3-methoxyacetophenone

| Conditions | Yield |
|---|---|
| With potassium phosphate; tetra-(n-butyl)ammonium iodide In N,N-dimethyl-formamide at 20℃; for 30h; Inert atmosphere; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; for 2h; | 100% |
| In pyridine at 5℃; for 0.166667h; | 99% |
-
-
4897-84-1
Methyl 4-bromobutyrate

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
175281-79-5
methyl 4-(4-acetyl-2-methoxyphenoxy)butanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 16h; Ambient temperature; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 16h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide | |
| With potassium carbonate In N,N-dimethyl-formamide for 16h; Inert atmosphere; |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
188891-12-5
(4-acetyl-2-methoxy-phenoxy)acetic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 48h; | 100% |
| Stage #1: 1-(3-methoxy-4-hydroxyphenyl)ethanone With sodium hydroxide In methanol at 20℃; for 15h; Stage #2: bromoacetic acid tert-butyl ester In methanol for 15h; Reflux; | 100% |
| With potassium carbonate In water; N,N-dimethyl-formamide at 0℃; | 99% |
-
-
2969-81-5
4-bromoethylbutanoate

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
174884-21-0
ethyl 4-(4-acetyl-2-methoxyphenoxy) butanoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 50℃; for 19h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 50℃; for 19h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 50℃; for 19h; | 100% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
149105-13-5
4-acetyl-2-methoxyphenyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide; acetonitrile at 20℃; for 12h; | 100% |
-
-
64-19-7
acetic acid

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
54771-60-7
4-acetyl-2-methoxyphenyl acetate

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 20℃; for 3h; | 100% |
-
-
4897-84-1
Methyl 4-bromobutyrate

-
-
584-08-7
potassium carbonate

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
175281-79-5
methyl 4-(4-acetyl-2-methoxyphenoxy)butanoate

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide | 100% |
| In water; N,N-dimethyl-formamide | 100% |

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
163105-90-6
2-methoxypyridin-3-yl-boronic acid

-
-
943152-72-5
1-[3-methoxy-4-(2-methoxy-pyridin-3-yl)-phenyl]-ethanone

| Conditions | Yield |
|---|---|
| Stage #1: 1-(3-methoxy-4-hydroxyphenyl)ethanone With N,N-phenylbistrifluoromethane-sulfonimide; potassium carbonate In tetrahydrofuran at 120℃; for 0.1h; Irradiation; Stage #2: 2-methoxypyridin-3-yl-boronic acid; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 120℃; for 0.166667h; | 100% |

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at -78℃; for 0.25h; Inert atmosphere; | 100% |
| With aluminium(III) iodide; carbonic acid dimethyl ester In acetonitrile at 80℃; for 18h; Reagent/catalyst; | 97% |
| With aluminium(III) iodide; carbonic acid dimethyl ester In acetonitrile at 80℃; for 18h; Reagent/catalyst; | 97% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 21h; | 100% |
-
-
100-39-0
benzyl bromide

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
1835-11-6
4-benzyloxy-3-methoxyacetophenone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1h; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; | 99% |
-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
74-88-4
methyl iodide

-
-
1131-62-0
1-(3,4-dimethoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 1h; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 4h; | 93.8% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 4h; | 90.7% |
| With sodium hydroxide In N,N-dimethyl acetamide at 25℃; Rate constant; | |
| With potassium carbonate In acetone at 20℃; for 4h; |

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
149105-13-5
4-acetyl-2-methoxyphenyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 99% |
-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
140690-56-8
2,4-bis(trifluoromethyl)benzyl bromide

-
-
885597-59-1
1-[4-(2,4-bis-trifluoromethyl-benzyloxy)-3-methoxy-phenyl]-ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60 - 70℃; for 15h; | 99% |

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
66338-49-6
3-methoxy-4-benzenesulphonyloxy-acetophenone

| Conditions | Yield |
|---|---|
| With sodium hydroxide | 99% |
| With sodium hydroxide In water | 99% |
-
-
106-95-6
allyl bromide

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
116218-80-5
1-(4-(allyloxy)-3-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 70℃; | 98% |
| With potassium carbonate In acetone at 20℃; for 24h; | 98% |
| With potassium carbonate; acetone | |
| With potassium carbonate In acetone Reflux; | |
| With potassium carbonate In acetone for 3.16667h; Reflux; |
-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With N(CH2)3CHCPh2OBO(n-Oct) | 98% |
| With octanol; (S)-diphenylprolinol; dimethylsulfide borane complex In toluene at 34℃; | 98% |

| Conditions | Yield |
|---|---|
| With Triisopropoxymethan; cerium triflate In hexane at 25℃; for 3h; | 98% |
-
-
106-96-7
propargyl bromide

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
956597-80-1
1-(3-methoxy-4-(prop-2-yn-1-yloxy)phenyl)ethan-1-one

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 25℃; for 12h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 16h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 20℃; for 72h; Claisen-Schmidt Condensation; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 20℃; for 72h; Claisen-Schmidt Condensation; Inert atmosphere; | 98% |
| With potassium hydroxide In ethanol at 0 - 20℃; for 73h; Claisen-Schmidt Condensation; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 20℃; for 72h; Claisen-Schmidt Condensation; Inert atmosphere; | 98% |
| With potassium hydroxide In ethanol at 0 - 20℃; for 73h; Claisen-Schmidt Condensation; Inert atmosphere; | 61% |
-
-
14371-10-9
(E)-3-phenylpropenal

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 20℃; Claisen-Schmidt Condensation; | 98% |
-
-
108-24-7
acetic anhydride

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
54771-60-7
4-acetyl-2-methoxyphenyl acetate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane Ambient temperature; | 97% |
| In pyridine for 18h; Acetylation; | |
| With pyridine; dmap In dichloromethane |
-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In 2-methylbenzenesulphochloride; water | 97% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; | 97% |
-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
112-89-0
1-Bromooctadecane

-
-
1603965-07-6
1-(3-methoxy-4-(octadecyloxy)phenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide; acetone at 60℃; | 96.7% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
132255-78-8
4'-tert-butyldimethylsilyloxy-3'-methoxyacetophenone

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 3h; Ambient temperature; | 96% |
| With 1H-imidazole at 20℃; | 95% |
-
-
107-30-2
chloromethyl methyl ether

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
143418-77-3
1-<3-methoxy-4-(methoxymethoxy)phenyl>ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; | 96% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 6h; | 62% |
| With sodium hydride 1.) THF, DMF, RT, 3 h, 2.) THF, DMF, RT, 2 h; Yield given. Multistep reaction; |
-
-
75-03-6
ethyl iodide

-
-
498-02-2
1-(3-methoxy-4-hydroxyphenyl)ethanone

-
-
75665-89-3
1-<(4-ethoxy-3-methoxy)phenyl>ethan-1-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 2h; | 96% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; | 96% |
| With potassium carbonate In acetone for 2h; Heating; | 80% |
Acetovanillone Chemical Properties
Molecular Structure of Acetovanillone (CAS NO.498-02-2):
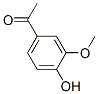
IUPAC Name: 1-(4-hydroxy-3-methoxyphenyl)ethanone
CAS: 498-02-2
Molecular Formula: C9H10O3
Molecular Weight: 166.17
EINECS: 207-854-5
Melting point: 112-115 °C(lit.)
Boiling point: 263-265 °C17 mm Hg(lit.)
Flash point: >230 °F
Water Solubility: soluble in hot water
Merck: 14,741
BRN: 637373
Index of Refraction: 1.537
Molar Refractivity: 44.84 cm3
Molar Volume: 143.3 cm3
Polarizability: 17.77*10-24cm3
Surface Tension: 41.5 dyne/cm
Density: 1.158 g/cm3
Enthalpy of Vaporization: 55.89 kJ/mol
Vapour Pressure: 0.000759 mmHg at 25°C
InChI
InChI=1/C9H10O3/c1-6(10)7-3-4-8(11)9(5-7)12-2/h3-5,11H,1-2H3
Smiles
c1(cc(ccc1O)C(C)=O)OC
Product Categories: Fine chemical & intermediates; Aromatic Acetophenones & Derivatives (substituted); pharmacetical; Adehydes, Acetals & Ketones; Anisoles, Alkyloxy Compounds & Phenylacetates; Phenols
Acetovanillone Uses
Acetovanillone (CAS NO.498-02-2) is a plant-derived, cartilage-saving drug which might be mainly used in the treatment of rheumatoid arthritis.
Acetovanillone Toxicity Data With Reference
| 1. | mmo-smc 400 mg/L | MUREAV Mutation Research. 119 (1983),272. | ||
| 2. | orl-mus LD50:9 g/kg | BCTKAG Bromatologia i Chemia Toksykologiczna. 14 (1984),301. | ||
| 3. | ipr-mus LD50:650 mg/kg | JMCMAR Journal of Medicinal Chemistry. 7 (1964),178. |
Acetovanillone Consensus Reports
Reported in EPA TSCA Inventory.
Acetovanillone Safety Profile
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. Mutation data reported. When heated to decomposition it emits acrid smoke and fumes.
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 2
HS Code: 29145000
Acetovanillone Specification
Acetovanillone (CAS NO.498-02-2) is also named as 4-Hydroxy-3-methoxyacetophenone ; Apocynin ; Acetovanillone~Apocynin ; 4-Hydroxy-3-methoxybenzoic acid ; 3-Methoxy-4-Hydroxy Acetophenone and so on. It is usually light yellow to yellow solid with faint vanilla odor.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View