-
Name
Anisic acid
- EINECS 202-818-5
- CAS No. 100-09-4
- Article Data1469
- CAS DataBase
- Density 1.208 g/cm3
- Solubility Soluble in alcohol, ether, chloroform, slightly soluble in water, insoluble in cold water
- Melting Point 181-186 °C
- Formula C8H8O3
- Boiling Point 278.305 °C at 760 mmHg
- Molecular Weight 152.15
- Flash Point 115.46 °C
- Transport Information 25kgs
- Appearance White powder
- Safety 24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms p-Anisicacid (6CI,7CI,8CI);4-Anisic acid;4-Methoxybenzoic acid;Draconicacid;NSC 32742;NSC 7926;p-Methoxybenzoic acid;
- PSA 46.53000
- LogP 1.39340
Synthetic route

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; oxygen; triphenylphosphine In ethyl acetate for 10h; fluorescent irradiation; | 100% |
| With oxygen; cobalt(II) acetate; manganese(II) acetate; 1N,3N,5N-trihydroxy-1,3,5-triazin-2,4,6[1H,3H,5H]-trione In acetic acid at 80℃; for 6h; | 99% |
| With Iron(III) nitrate nonahydrate; dihydrogen peroxide; oxygen; manganese(II) acetate; acetic acid at 25℃; under 30003 Torr; for 0.0266111h; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; oxygen In acetonitrile at 20℃; for 5h; UV-irradiation; | 100% |
| With [Cu2C6H4(CHNCH2CH2N(CH2C5H4N)2)2](2+)*2ClO4(1-)=C36H38Cu2N8(ClO4)2; oxygen In acetone at -90.16℃; | 100% |
| With cobalt(II) 2,9,16,23-phthalocyanine tetrasulfonic acid In water; acetonitrile at 20℃; under 760.051 Torr; for 150h; Reagent/catalyst; Solvent; UV-irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With Au NCs/TiO2; oxygen; sodium hydroxide In water at 80℃; under 7500.75 Torr; for 6h; Autoclave; Green chemistry; | 100% |
| With palladium 10% on activated carbon; water; sodium hydroxide at 80℃; under 600.06 Torr; for 6h; | 100% |
| With gold oxide; oxygen; copper(II) oxide; sodium hydroxide; silver(l) oxide In water at 40℃; under 750.075 Torr; for 16h; | 100% |
-
-
67-56-1
methanol

-
-
145224-20-0
4-Methoxy-benzoic acid 4-cyano-naphthalen-1-ylmethyl ester

-
A
-
36062-93-8
1-cyano-4-methylnaphthalene

-
B
-
112929-94-9
1-cyano-4-(methoxymethyl)naphthalene

-
C
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| Irradiation; | A 5 % Chromat. B 66 % Chromat. C 100% D 22 % Chromat. |


| Conditions | Yield |
|---|---|
| With formic acid; triethylamine In acetonitrile at 80℃; for 1h; Inert atmosphere; | 100% |
| With sodium hydrogen telluride; acetic acid In ethanol for 2h; Heating; | 95% |
| With toluene-4-sulfonic acid for 0.0666667h; microwave irradiation; | 82% |
| With iodine; dimethyl sulfoxide for 0.5h; Heating; | 75% |
-
-
145224-20-0
4-Methoxy-benzoic acid 4-cyano-naphthalen-1-ylmethyl ester

-
A
-
36062-93-8
1-cyano-4-methylnaphthalene

-
B
-
112929-94-9
1-cyano-4-(methoxymethyl)naphthalene

-
C
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| In methanol Irradiation; | A 5 % Chromat. B 66 % Chromat. C 100% D 22 % Chromat. |
| In methanol Irradiation; | A 39 % Chromat. B 60 % Chromat. C 61% D 2 % Chromat. |

-
-
52509-81-6
potassium 4-methoxybenzoate

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| Hydrolysis; | 100% |

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 0.166667h; Microwave irradiation; chemoselective reaction; | 99% |
| Stage #1: methyl 4-methoxybenzoate With sodium hydroxide In methanol at 60 - 65℃; for 3h; Stage #2: With hydrogenchloride In methanol; water | 99.67% |
| With pyridine; iodine; aluminium In acetonitrile at 80℃; for 18h; Solvent; Reagent/catalyst; Temperature; | 98% |

| Conditions | Yield |
|---|---|
| With copper(II) nitrate trihydrate; oxygen In acetonitrile at 120℃; under 4500.45 Torr; for 10h; Autoclave; | 99% |
| With oxygen; copper(II) nitrate In acetonitrile at 120℃; under 4500.45 Torr; for 10h; | 99% |
| With copper(l) iodide; hydroxylamine hydrochloride; oxygen In dimethyl sulfoxide at 100℃; for 8h; Solvent; Reagent/catalyst; Temperature; | 95% |

| Conditions | Yield |
|---|---|
| With 3,4-benzo-1,1,2,2-tetraethyl-1,2-disilacyclobut-3-ene; cesium fluoride In N,N-dimethyl-formamide at 0 - 20℃; under 760.051 Torr; for 2h; Reagent/catalyst; | 99% |
| With tetraethylammonium tosylate; triphenylphosphine; tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide Pt anode/Pt cathode; electrolysis with 2.5 mA/cm2; | 82% |
| With copper(l) iodide; N,N,N,N,-tetramethylethylenediamine; diethylzinc In dimethyl sulfoxide at 70℃; under 760.051 Torr; | 60% |

| Conditions | Yield |
|---|---|
| With water; potassium carbonate In acetonitrile at 100℃; under 3750.38 Torr; for 0.0161111h; | 99% |
| With dichloro bis(acetonitrile) palladium(II); potassium hydroxide; sodium dodecyl-sulfate In water; toluene; butan-1-ol at 50℃; under 760 Torr; for 4h; | 96% |
| With potassium hydroxide; amphiphilic resin-supported phosphine-palladium; water at 25℃; under 760 Torr; for 12h; Product distribution; Further Variations:; Reagents; hydroxycarbonylation; | 96% |
-
-
24318-43-2
4-methoxybenzyl 4-methoxybenzoate

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With proton-exchanged montmorillonite In dichloromethane at 20℃; | 99% |
| With trichlorophosphate In 1,2-dichloro-ethane at 20℃; for 1h; | 88% |
| With oxalyl dichloride In 1,2-dichloro-ethane at 20℃; for 2.66667h; | 75% |
-
-
19513-80-5
2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)ethan-1-one

-
A
-
90-05-1
2-methoxy-phenol

-
B
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) nitrate In acetonitrile at 120℃; under 4500.45 Torr; for 10h; | A n/a B 99% |
| With oxygen; copper diacetate; triethylamine In dimethyl sulfoxide at 20℃; for 12h; Sealed tube; | A 93% B 83% |
| With water; oxalic acid at 100℃; for 24h; Reagent/catalyst; | A 21 mg B 73% |
-
-
140455-39-6
2-(2,6-dimethoxyphenoxy)-1-(4-methoxyphenyl)ethan-1-one

-
A
-
91-10-1
1,3-dimethoxy-2-hydroxy-benzene

-
B
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) nitrate In acetonitrile at 120℃; under 4500.45 Torr; for 10h; | A n/a B 99% |

| Conditions | Yield |
|---|---|
| With Ximenia american In aq. phosphate buffer; water at 30℃; for 72h; pH=7; Enzymatic reaction; | A n/a B 98% |
| Stage #1: 4-methoxy-benzaldehyde With potassium hydroxide for 0.0833333h; Cannizzaro Reaction; Milling; Inert atmosphere; Sealed tube; Green chemistry; Stage #2: With hydrogenchloride In water Green chemistry; | A 95% B 94% |
| With sodium hydroxide In water at 15℃; for 2h; Cannizzaro Reaction; | A 91% B n/a |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; dicobalt octacarbonyl In sodium hydroxide; benzene at 65℃; for 2h; Irradiation; variation of reaction medium; | 98% |
| With tetrabutylammomium bromide; dicobalt octacarbonyl In sodium hydroxide; benzene at 65℃; for 2h; Irradiation; variation of reaction medium; | 98% |
| With sodium hydroxide; tetrabutylammomium bromide; dicobalt octacarbonyl In water; benzene at 65℃; under 760 Torr; for 2h; Irradiation; | 94% |
-
-
114050-46-3
N-(4-methoxybenzoyloxy)-pyridine-2(1H)-thione

-
-
75-66-1
2-methylpropan-2-thiol

-
A
-
24367-44-0
2-(2-tert-butyldisulfanyl)pyridine

-
B
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| In dichloromethane Irradiation; | A 86% B 98% |


| Conditions | Yield |
|---|---|
| With 40% potassium fluoride/alumina for 0.0666667h; Microwave irradiation; Neat (no solvent); | A 98% B 92% |
-
-
213468-59-8
S-methyl 3-oxo-3-(4-methoxylphenyl)propanedithioate

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 100℃; for 10h; Reagent/catalyst; Reflux; chemoselective reaction; | 98% |

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 0.5h; | 98% |

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate In nitromethane at 45 - 50℃; for 18h; | 97% |
| With zinc dibromide In dichloromethane for 6h; dealkylation; | 93% |
| With toluene-4-sulfonic acid for 0.0666667h; microwave irradiation; | 92% |
| With silica gel In toluene for 7h; Heating; | 68% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methyltrioxorhenium(VII); magnesium sulfate In acetonitrile for 3.5h; Heating; | 97% |
| With diphenyl diselenide; dihydrogen peroxide In water; acetonitrile at 20℃; for 24h; | 88% |
| With oxygen; potassium hydroxide at 20℃; Schlenk technique; chemoselective reaction; | 87% |
-
-
124-38-9
carbon dioxide

-
-
213596-33-9
2-(4-methoxyphenyl)-5,5-dimethyl-1,3,2-dioxaborolane

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; copper(l) chloride; 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride In tetrahydrofuran at 70℃; under 760.051 Torr; for 24h; | 97% |
| Stage #1: carbon dioxide; 2-(4-methoxyphenyl)-5,5-dimethyl-1,3,2-dioxaborolane With [Ni(N,N'-bis[2,6-bis(diphenylmethyl)-4-methylphenyl]imidazole-2-ylidene)(allyl)Cl]; potassium tert-butylate In toluene at 100℃; under 760.051 Torr; for 15h; Schlenk technique; Inert atmosphere; Stage #2: With hydrogenchloride In water; ethyl acetate; toluene at 20℃; Solvent; Temperature; Reagent/catalyst; | 96% |
| With 1,3-bis-(diphenylphosphino)propane; cesium fluoride; [Rh(OH)(cod)]2 In 1,4-dioxane at 60℃; | 95% |
-
-
57297-25-3
1-(4-methoxyphenyl)-2-(4-methylphenyl)ethanone

-
A
-
99-94-5
p-Toluic acid

-
B
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With oxygen; potassium hydroxide at 20℃; Schlenk technique; chemoselective reaction; | A 93% B 97% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium hydride In N,N-dimethyl acetamide at 60℃; for 2h; Inert atmosphere; | 96% |
| With palladium diacetate; sodium hydride In N,N-dimethyl acetamide at 50℃; for 5h; Inert atmosphere; | 96% |
| With aluminum oxide for 0.333333h; microwave irradiation; | 89% |
| With methanol; sodium tetrahydroborate; nickel(II) chloride hexahydrate at 20℃; for 0.333333h; chemoselective reaction; | 88% |
-
-
696-62-8
para-iodoanisole

-
-
18414-58-9
diphenylmethylsilanecarboxylic acid

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With potassium trimethylsilonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; bis(dibenzylideneacetone)-palladium(0) In toluene at 40℃; for 0.333333h; Reagent/catalyst; Solvent; Time; Temperature; | 96% |
-
-
14660-45-8
potassium monomethylcarbonate

-
-
213596-33-9
2-(4-methoxyphenyl)-5,5-dimethyl-1,3,2-dioxaborolane

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With chloro[1,3-bis(2,6-di-i-propylphenyl)imidazol-2-ylidene]copper(I) In tetrahydrofuran at 70℃; for 16h; Inert atmosphere; Sealed tube; | 96% |
-
-
2729-19-3
2-(4-Fluorophenyl)-1-(4-methoxyphenyl)ethan-1-one

-
A
-
456-22-4
4-Fluorobenzoic acid

-
B
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With oxygen; potassium hydroxide at 20℃; Schlenk technique; chemoselective reaction; | A 95% B 96% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 4h; Reflux; | 100% |
| With sulfuric acid Fischer-Speier esterification method; Reflux; | 100% |
| With cobalt oxide nanoparticles Co/SBA-15 for 12h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 40℃; for 14h; Inert atmosphere; | 100% |
| With 4-methyl-morpholine; 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride for 3h; | 93% |
| With thionyl chloride at 0℃; for 5h; Reflux; | 90% |

| Conditions | Yield |
|---|---|
| With thionyl chloride Reflux; | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 20℃; for 2h; Reflux; | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 35℃; for 1h; | 100% |
-
-
98-88-4
benzoyl chloride

-
-
100-09-4
4-methoxybenzoic acid

-
-
58618-94-3
benzoic 4-methoxybenzoic anhydride

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; | 100% |
| cobalt(II) chloride In dichloromethane; acetonitrile at 40℃; | 89% |

| Conditions | Yield |
|---|---|
| With diphosphorus tetraiodide In tetrachloromethane; dichloromethane Heating; | 100% |
| With dmap; 2-chloro-1-(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-henicosafluorododecyl)pyridinium trifluoromethanesulfonate; triethylamine In N,N-dimethyl-formamide at 20℃; for 1h; | 100% |
| With dmap; 2-chloro-1-(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,12-henicosafluorododecyl)pyridinium trifluoromethanesulfonate; triethylamine In N,N-dimethyl-formamide at 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine; hydrogen fluoride; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 3h; | 100% |
| With sodium fluoride; ethanaminium,N-(difluoro-λ4-sulfanylidene)-N-ethyl-,tetrafluoroborate at 20℃; for 24h; Inert atmosphere; | 99% |
| With potassium 2-(difluoro(trifluoromethoxy)methoxy)-2,2-difluoroacetate In acetonitrile at 80℃; for 1h; Schlenk technique; Sealed tube; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile for 1.5h; Heating; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 50℃; | 90% |
| With 18-crown-6 ether; potassium carbonate In tetrahydrofuran for 21h; Inert atmosphere; Reflux; |
-
-
75-84-3
2,2-dimethyl-propanol-1

-
-
100-09-4
4-methoxybenzoic acid

-
-
3581-72-4
neopentyl 4-methoxybenzoate

| Conditions | Yield |
|---|---|
| With sulfuric acid In toluene | 100% |
-
-
64-04-0
phenethylamine

-
-
100-09-4
4-methoxybenzoic acid

-
-
6346-07-2
4-methoxy-N-(2-phenylethyl)benzamide

| Conditions | Yield |
|---|---|
| With 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride In methanol at 20℃; for 3h; | 100% |
| With 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride In tetrahydrofuran at 20℃; for 3h; Condensation; | 95% |
| With 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride In tetrahydrofuran for 4h; | 94% |
-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
100-09-4
4-methoxybenzoic acid

-
-
52898-49-4
4,N-dimethoxy-N-methylbenzamide

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxybenzoic acid With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide at 0℃; Stage #2: N,O-dimethylhydroxylamine*hydrochloride With triethylamine In dichloromethane; N,N-dimethyl-formamide at 20℃; Further stages.; | 100% |
| Stage #1: 4-methoxybenzoic acid With triethylamine; trichloromethyl chloroformate In dichloromethane at 0℃; Stage #2: N,O-dimethylhydroxylamine*hydrochloride In dichloromethane at 25℃; for 1h; | 92% |
| Stage #1: 4-methoxybenzoic acid With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide; triethylamine In tetrahydrofuran; ethyl acetate at 0 - 5℃; for 0.166667h; Inert atmosphere; Stage #2: N,O-dimethylhydroxylamine*hydrochloride In tetrahydrofuran; ethyl acetate at 0 - 25℃; for 1h; Inert atmosphere; | 92% |
-
-
1029720-98-6
ethyl 4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazine-2-carboxylate

-
-
100-09-4
4-methoxybenzoic acid

-
-
1338563-35-1
ethyl 5-(4-methoxybenzoyl)-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazine-2-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxybenzoic acid With triethylamine; fluoro-N,N,N',N'-tetramethylformamidinium hexafluorophosphate In N,N-dimethyl-formamide for 0.25h; Stage #2: ethyl 4,5,6,7-tetrahydropyrazolo[1,5-a]pyrazine-2-carboxylate In N,N-dimethyl-formamide at 20℃; for 5h; | 100% |
| With triethylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate |
-
-
188111-79-7
tert-butyl (3R)3-aminopiperidine-1-carboxylate

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In 1,4-dioxane; N,N-dimethyl-formamide at 20℃; for 3h; | 100% |
-
-
41879-37-2
t-butyldimethylsilyl amine

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 0.5h; Green chemistry; | 100% |
-
-
711-79-5
1-hydroxy-2-acetonaphthone

-
-
100-09-4
4-methoxybenzoic acid

-
-
934163-66-3
2-acetylnaphthalen-1-yl 4-methoxybenzoate

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxybenzoic acid With thionyl chloride; N,N-dimethyl-formamide In dichloromethane at 40℃; for 0.666667h; Stage #2: 1-hydroxy-2-acetonaphthone With pyridine In dichloromethane at 60℃; for 1h; | 100% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; |
-
-
75-59-2
tetramethyl ammoniumhydroxide

-
-
100-09-4
4-methoxybenzoic acid

-
-
111536-98-2
tetramethylammonium 4-methoxybenzoate

| Conditions | Yield |
|---|---|
| With water In ethanol at 0 - 20℃; for 0.0833333h; | 100% |
-
-
10267-21-7
13(S)-labdan-8α,15-diol

-
-
100-09-4
4-methoxybenzoic acid

| Conditions | Yield |
|---|---|
| With dmap; diisopropyl-carbodiimide In dichloromethane at 20℃; Cooling with ice; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxybenzoic acid With 1,3,5-trichloro-2,4,6-triazine; potassium carbonate; triphenylphosphine at 20℃; for 0.166667h; Stage #2: benzylamine at 20℃; for 0.333333h; | 99% |
| Stage #1: 4-methoxybenzoic acid With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0℃; for 0.166667h; Stage #2: benzylamine In N,N-dimethyl-formamide at 20℃; for 0.916667h; Further stages.; | 97% |
| With 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride In methanol for 3h; | 96% |
-
-
100-39-0
benzyl bromide

-
-
100-09-4
4-methoxybenzoic acid

-
-
6316-54-7
4-methoxy-benzoic acid benzyl ester

| Conditions | Yield |
|---|---|
| With N(Et)4(1+)*(2-pyrrolidone-anion) In N,N-dimethyl-formamide for 1h; Ambient temperature; | 99% |
-
-
74684-57-4
1a,9b-dihydro-1H-phenanthro<9,10-b>azirine

-
-
100-09-4
4-methoxybenzoic acid

-
-
117608-96-5
4-methoxy-N-(phenanthrene-9-yl)benzamide

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane for 3h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxybenzoic acid With 1,3,5-trichloro-2,4,6-triazine; potassium carbonate In tetrahydrofuran for 0.0166667h; Milling; Stage #2: With ammonium thiocyanate In tetrahydrofuran for 0.0833333h; Milling; | 99% |
| With pyridine; urea for 0.00833333h; microwave irradiation; | 90% |
| Stage #1: 4-methoxybenzoic acid With thionyl chloride In tetrahydrofuran at 50℃; for 1h; Stage #2: With ammonium hydroxide In tetrahydrofuran at 0℃; for 0.0833333h; | 84% |
Anisic acid Specification
The Anisic acid with CAS registry number of 100-09-4 is also known as Benzoic acid,4-methoxy-. The IUPAC name is 4-Methoxybenzoic acid. It belongs to product categories of Liquid Crystal intermediates; Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts; Organic Acids; Absolute Configuration Determination (Exciton Chirality CD Method); Enantiomer Excess & Absolute Configuration Determination; Exciton Chirality CD Method (for Hydroxyl Groups); Analytical Chemistry;Benzoic Acids (Building Blocks for Liquid Crystals); Building Blocks for Liquid Crystals; Functional Materials. Its EINECS registry number is 202-818-5. In addition, the formula is C8H8O3 and the molecular weight is 152.15. This chemical is a white powder and soluble in alcohol, ether, chloroform, slightly soluble in water, insoluble in cold water. What's more, it should be sealed in ventilated, cool and dry place away from fore, heat.
Physical properties about Anisic acid are: (1)ACD/LogP: 1.78; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1; (4)ACD/LogD (pH 7.4): -1; (5)ACD/BCF (pH 5.5): 2; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 24; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 46.53Å2; (13)Index of Refraction: 1.546; (14)Molar Refractivity: 39.861 cm3; (15)Molar Volume: 125.965 cm3; (16)Polarizability: 15.802×10-24cm3; (17)Surface Tension: 44.9 dyne/cm; (18)Density: 1.208 g/cm3; (19)Flash Point: 115.46 °C; (20)Enthalpy of Vaporization: 54.601 kJ/mol; (21)Boiling Point: 278.305 °C at 760 mmHg; (22)Vapour Pressure: 0.002 mmHg at 25 °C.
Preparation of Anisic acid: it is prepared by reaction of hydroxybenzoic acid with dimethyl sulfate. Equation is as follows:
C7H6O3 + (CH3)2SO4 → C8H8O3
Uses of Anisic acid: it is used as a preservative and raw materials of medicine and fragrance. What's more, it is used to produce 4-methoxy-benzoic acid methyl ester by esterification reaction with methanol. The reaction occurs with reagents 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium Cl, N-methylmorpholine at the temperature of 50 °C for 6 hours. The yield is about 88%.
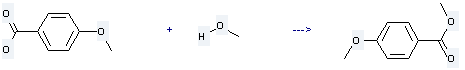
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing. Avoid contact with skin and eyes. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: COC1=CC=C(C=C1)C(=O)O
2. InChI: InChI=1S/C8H8O3/c1-11-7-4-2-6(3-5-7)8(9)10/h2-5H,1H3,(H,9,10)
3. InChIKey: ZEYHEAKUIGZSGI-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | 400mg/kg (400mg/kg) | Cesko-Slovenska Farmacie. Vol. 31, Pg. 236, 1982. |
Related Products
- Anisic acid
- Anisic alcohol
- 10009-70-8
- 1000981-74-7
- 1000994-94-4
- 1000998-59-3
- 1001020-14-9
- 1001020-17-2
- 1001050-24-3
- 10010-67-0
- 100-10-7
- 10010-93-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View