-
Name
Clarithromycin
- EINECS 1806241-263-5
- CAS No. 81103-11-9
- Article Data13
- CAS DataBase
- Density 1.184 g/cm3
- Solubility 99.48mg/L(20 oC)
- Melting Point 217-220 °C
- Formula C38H69NO13
- Boiling Point 805.478 °C at 760 mmHg
- Molecular Weight 747.965
- Flash Point 440.937 °C
- Transport Information
- Appearance Colourless crystalline needles
- Safety 26-36
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Adel;Mabicrol;TE 031;Klaricid;Biaxin (TN);(3R,4S,5S,6R,7R,9R,11R,12R,13R,14R)-6-(4-dimethylamino-3-hydroxy-6-methyl-oxan-2-yl)oxy-14-ethyl-12,13-dihydroxy-4-(5-hydroxy-4-methoxy-4,6-dimethyl-oxan-2-yl)oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione;Clarithromycine [INN-French];Clacine;Trovafloxacin & Clarithromycin;6-o-Methoxyerythromycin;(3R,4S,6R,7R,9R,11R,12R,13R,14R)-6-[(2S,3R,4S,6R)-4-dimethylamino-3-hydroxy-6-methyl-oxan-2-yl]oxy-14-ethyl-12,13-dihydroxy-4-[(2S,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyl-oxan-2-yl]oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione;Abbott-56268;Clarith;Klacid;Claritromicina [INN-Spanish];Klaciped;6-O-Methylerythromycin A;Kofron;Heliclar;Klarin;TE-031;TE031;Maclar;Klaricid Pediatric;Biaxin;(3R,4S,5S,6R,7R,9R,11R,12R,13R,14R)-6-[(2S,3R,4S,6R)-4-dimethylamino-3-hydroxy-6-methyl-oxan-2-yl]oxy-14-ethyl-12,13-dihydroxy-4-[(2S,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyl-oxan-2-yl]oxy-7-methoxy-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione;Helas;Veclam;Klarid;Clathromycin;Zeclar;Clarithromycinum [INN-Latin];Claribid;Klax;Mavid;
- PSA 182.91000
- LogP 2.43970
Synthetic route
-
-
118074-07-0
6-O-methylerythromycin A N-oxide

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Stage #1: 6-O-methylerythromycin A N-oxide With tin(ll) chloride In isopropyl alcohol at 30 - 40℃; for 2h; Stage #2: With sodium hydrogencarbonate In water; isopropyl alcohol | 96% |
| With bis(tri-n-butyltin) In tetrahydrofuran for 24h; Heating / reflux; | 95% |
| With hydrogen; W4 Raney-nickel In ethanol at 40 - 50℃; for 3h; | 92% |
| With potassium hydroxide; nickel-aluminum alloy In methanol; water at 35 - 40℃; for 3.5h; | 92% |
| Stage #1: 6-O-methylerythromycin A N-oxide With tin(ll) chloride In dichloromethane at 20℃; for 1.5h; Stage #2: With sodium carbonate In dichloromethane; water | 32% |
-
-
930287-49-3
clarythromycin 9-O-(2-pyrimidyl)oxime

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With sodium hydrogensulfite In ethanol; water at 80℃; for 6h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 6-O-methylerythromycin A N-oxide With sodium hydrogensulfite In ethanol; water at 20℃; for 1h; Stage #2: With sodium carbonate In ethanol; water Stage #3: formaldehyd With formic acid; sodium carbonate more than 3 stages; | 92% |
| Stage #1: 6-O-methylerythromycin A N-oxide With sodium hydrogensulfite In ethanol; water at 20℃; for 1h; Stage #2: With sodium carbonate In ethanol; water Stage #3: formaldehyd With formic acid; ammonia more than 3 stages; | 30% |
-
-
103450-87-9, 127253-05-8, 127253-06-9
clarithromycin 9-oxime

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With sodium disulfide In ethanol Heating; | 68% |
-
-
130034-54-7
9-deoxo-9-imino-6-O-methylerythromycin A

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With water In methanol at 23℃; pH 12.4 or pH 7; | |
| With hydrogenchloride; water In ethanol pH=5 - 6; |

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With sodium metabisulfite; formic acid In ethanol for 0.5h; Hydrolysis; Heating; | 4.32 g |
-
-
930287-46-0
6-(4-dimethylamino-3-hydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-14-ethyl-7,12,13-trihydroxy-4-(5-hydroxy-4-methoxy-4,6-dimethyl-tetrahydro-pyran-2-yloxy)-3,5,7,9,11,13-hexamethyl-oxacyclotetradecane-2,10-dione 10-(O-pyrimidin-2-yl-oxime)

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / trimethylsilyl-imidazole / CH2Cl2 / 2 h / 0 °C 2: 83 percent / KOH / dimethylsulfoxide 3: 97 percent / HCO2H / ethanol; H2O 4: 95 percent / sodium hydrogen sulfite / ethanol; H2O / 6 h / 80 °C View Scheme |
-
-
930287-48-2
C48H88N4O13Si2

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 97 percent / HCO2H / ethanol; H2O 2: 95 percent / sodium hydrogen sulfite / ethanol; H2O / 6 h / 80 °C View Scheme |
-
-
930287-47-1
2',4''-O-bistrimethylsilyl erythromycin A 9-O-(2-pyrimydyl)oxime

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 83 percent / KOH / dimethylsulfoxide 2: 97 percent / HCO2H / ethanol; H2O 3: 95 percent / sodium hydrogen sulfite / ethanol; H2O / 6 h / 80 °C View Scheme |

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 93 percent / t-BuOK / 2 h / 80 °C 2: 90 percent / trimethylsilyl-imidazole / CH2Cl2 / 2 h / 0 °C 3: 83 percent / KOH / dimethylsulfoxide 4: 97 percent / HCO2H / ethanol; H2O 5: 95 percent / sodium hydrogen sulfite / ethanol; H2O / 6 h / 80 °C View Scheme |
-
-
127253-06-9
6-methoxyerythromycin A 9-oxime

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 25 percent / Bu3P / tetrahydrofuran; dioxane 2: H2O; HCl / ethanol / pH 5 - 6 View Scheme | |
| Multi-step reaction with 2 steps 1.1: PySeSePy; Me3P / tetrahydrofuran / 0.17 h / 0 °C 1.2: 80 percent / H2O / pH 10 2.1: H2O; HCl / ethanol / pH 5 - 6 View Scheme |
-
-
111321-02-9
erythromycin A 9-(E)-oxime

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / Et3N / dimethylformamide / 1 h / 20 °C 2: 95.1 percent / 1-trimethylsilylimidazole / ethyl acetate / 1 h / 20 °C 3: 8.82 g / NaH / tetrahydrofuran; dimethylsulfoxide / 1 h / 0 - 5 °C 4: 4.32 g / formic acid; Na2S2O5 / ethanol / 0.5 h / Heating View Scheme |

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95.1 percent / 1-trimethylsilylimidazole / ethyl acetate / 1 h / 20 °C 2: 8.82 g / NaH / tetrahydrofuran; dimethylsulfoxide / 1 h / 0 - 5 °C 3: 4.32 g / formic acid; Na2S2O5 / ethanol / 0.5 h / Heating View Scheme |

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 8.82 g / NaH / tetrahydrofuran; dimethylsulfoxide / 1 h / 0 - 5 °C 2: 4.32 g / formic acid; Na2S2O5 / ethanol / 0.5 h / Heating View Scheme |
-
-
101666-67-5, 127182-43-8
6-O-methyl-N-demethylerythromycin A 9-oxime

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HCOOH / ethanol / Heating 2: 68 percent / Na2S2 / aq. ethanol / Heating View Scheme |
-
-
101670-80-8, 101757-72-6, 103530-45-6
C60H86N2O17

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2 / catalytic hydrogenation 2: HCOOH / ethanol / Heating 3: 68 percent / Na2S2 / aq. ethanol / Heating View Scheme |
-
-
101666-32-4, 101757-71-5, 103530-88-7
C59H84N2O17

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 76 percent / KOH / dimethylsulfoxide; tetrahydrofuran 2: H2 / catalytic hydrogenation 3: HCOOH / ethanol / Heating 4: 68 percent / Na2S2 / aq. ethanol / Heating View Scheme |
-
-
101666-16-4, 101757-70-4, 103530-44-5
C52H78N2O17

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: CHCl3 / isomerization 2: 82 percent / NaH / dimethylformamide 3: 76 percent / KOH / dimethylsulfoxide; tetrahydrofuran 4: H2 / catalytic hydrogenation 5: HCOOH / ethanol / Heating 6: 68 percent / Na2S2 / aq. ethanol / Heating View Scheme |
-
-
101666-16-4, 101757-70-4, 103530-44-5
C52H78N2O17

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 82 percent / NaH / dimethylformamide 2: 76 percent / KOH / dimethylsulfoxide; tetrahydrofuran 3: H2 / catalytic hydrogenation 4: HCOOH / ethanol / Heating 5: 68 percent / Na2S2 / aq. ethanol / Heating View Scheme |
-
-
81103-09-5
2'-O,3'-N-bis(benzyloxycarbonyl)-N-demethylerythromycin A

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: NH2OH*HCl, AcONa / methanol 2: CHCl3 / isomerization 3: 82 percent / NaH / dimethylformamide 4: 76 percent / KOH / dimethylsulfoxide; tetrahydrofuran 5: H2 / catalytic hydrogenation 6: HCOOH / ethanol / Heating 7: 68 percent / Na2S2 / aq. ethanol / Heating View Scheme | |
| Multi-step reaction with 6 steps 1: 75 percent / NH2OH*HCl, AcONa / methanol 2: 82 percent / NaH / dimethylformamide 3: 76 percent / KOH / dimethylsulfoxide; tetrahydrofuran 4: H2 / catalytic hydrogenation 5: HCOOH / ethanol / Heating 6: 68 percent / Na2S2 / aq. ethanol / Heating View Scheme |
-
-
103450-87-9
3-O-descladinosyl-9-oxime clarithromycin

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 0.78 g / ammonium acetate, 15percent aq. titanium(III) chloride / methanol / 6 h 2: H2O / methanol / 23 °C / pH 12.4 or pH 7 View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: (9-E)-deoxo-9-hydroximinoerythromycin A; methyl iodide With 2-Methoxypropene; Pyridine hydrobromide; potassium hexamethylsilazane In dichloromethane Stage #2: With formic acid; sodium hydrogensulfite In ethanol; dichloromethane at 82 - 84℃; |
-
-
791845-56-2
2',4-O-bis(trimethylsilyl)-6-O-methylerythromycin A 9-(E)-O-(1-isopropoxycyclohexyl)-oxime

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Stage #1: 2',4-O-bis(trimethylsilyl)-6-O-methylerythromycin A 9-(E)-O-(1-isopropoxycyclohexyl)-oxime With formic acid In ethanol; water at 40 - 45℃; for 3h; pH=2.5 - 3.5; Stage #2: With sodium hydrogensulfite In ethanol; water for 1h; Product distribution / selectivity; Heating / reflux; |
-
-
643726-97-0
clarithromycin acetate

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| at 120℃; for 4h; under vacuum; |

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 40℃; for 1h; pH=9 - 11; Product distribution / selectivity; |

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 40℃; for 1h; pH=9 - 11; Product distribution / selectivity; |

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 40℃; for 1h; pH=9 - 11; Product distribution / selectivity; |
-
-
119699-81-9
2',4"-O-bis (trimethylsilyl)-6-O-methylerythromycin A 9-[O-(1-methoxy-1-methylethyl)oxime]

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Stage #1: 2',4"-O-bis (trimethylsilyl)-6-O-methylerythromycin A 9-[O-(1-methoxy-1-methylethyl)oxime] With formic acid; sodium hydrogensulfite In denatured spirit for 2h; Heating / reflux; Stage #2: With sodium hydroxide In denatured spirit; water at 20℃; pH=10.5; Product distribution / selectivity; |
-
-
119685-39-1
6-O-methyl-2',4-bis(trimethylsilyl)-erythromycin A 9-O-(2-methoxyprop-2-yl)oxime

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| Stage #1: 6-O-methyl-2',4-bis(trimethylsilyl)-erythromycin A 9-O-(2-methoxyprop-2-yl)oxime With sodium metabisulfite; formic acid; water In ethanol at 25 - 63℃; for 10h; pH=3.8 - 4.1; Stage #2: With sodium hydroxide In ethanol; water at 30 - 35℃; for 0.5h; pH=10.5 - 11.0; Product distribution / selectivity; |
-
-
81103-11-9
clarithromycin

-
-
108-24-7
acetic anhydride

-
-
152235-55-7
2',4''-di-O-acetyl-6-O-methylerythromycin A

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 20℃; | 100% |
| With dmap; triethylamine In dichloromethane at 20℃; for 17h; | 99% |
| With dmap In dichloromethane at 20℃; for 18h; Inert atmosphere; | 99% |
-
-
81103-11-9
clarithromycin

-
-
108-24-7
acetic anhydride

-
-
103461-66-1
2'-O-acetyl-6-O-methylerythromycin A

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 48h; | 99% |
| With triethylamine at 20℃; | 95% |
| With triethylamine In dichloromethane at 20℃; for 12h; | 95% |
-
-
81103-11-9
clarithromycin

-
-
118058-75-6
3'-N,N-didemethyl-6-O-methylerythromycin A

| Conditions | Yield |
|---|---|
| With iodine; sodium acetate In methanol; water at 20℃; for 0.25h; UV-irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With 2-(dimethylamino)pyrimidine; triethylamine In N,N-dimethyl-formamide at 45℃; for 4h; | 98% |
| With dmap; triethylamine In ethyl acetate at 70℃; for 16h; | 97% |
| With dmap; triethylamine In dichloromethane | 55% |
-
-
81103-11-9
clarithromycin

-
-
118058-74-5
3-O-descladinosylclarithromycin

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 0.5h; | 96% |
| Stage #1: clarithromycin With hydrogenchloride In methanol; water at 20℃; Stage #2: With sodium hydroxide In methanol; water pH=10 - 11; | 96% |
| With hydrogenchloride In water at 20℃; for 3h; | 96% |
-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With hydrazinium monoacetate In methanol for 46h; Reflux; | 95.1% |
| With hydrazinium monoacetate In methanol for 46h; Reflux; | 70% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Inert atmosphere; | 94% |
-
-
4784-77-4, 29576-14-5, 39616-19-8
(E/Z)-crotyl bromide

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Inert atmosphere; | 93% |
-
-
81103-11-9
clarithromycin

-
-
101666-68-6
N-desmethylclarithromycin

| Conditions | Yield |
|---|---|
| Stage #1: clarithromycin With iodine; sodium acetate In methanol; water at 55 - 60℃; for 3h; Stage #2: With ammonia In methanol; chloroform; water | 92% |
| Stage #1: clarithromycin With iodine; sodium acetate In methanol; water at 55 - 60℃; for 3h; Stage #2: With ammonia In methanol; chloroform; water | 92% |
| With iodine; sodium acetate In methanol; water at 55 - 60℃; for 3h; | 92% |
-
-
81103-11-9
clarithromycin

-
-
118074-07-0
6-O-methylerythromycin A N-oxide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In methanol; water at 20℃; for 15h; | 92% |

| Conditions | Yield |
|---|---|
| With dmap In chloroform at 110℃; for 8h; Temperature; | 91% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Inert atmosphere; | 91% |
-
-
81103-11-9
clarithromycin

-
-
103450-87-9
3-O-descladinosyl-9-oxime clarithromycin

| Conditions | Yield |
|---|---|
| Stage #1: clarithromycin With hydroxylamine hydrochloride; sodium acetate In methanol at 75℃; for 12h; Stage #2: With hydrogenchloride In methanol; water at 75℃; for 5h; | 90% |
| With hydroxylamine hydrochloride; triethylamine In methanol for 24h; Heating; | 2.2 g |
| Stage #1: clarithromycin With hydroxylamine hydrochloride; sodium carbonate In methanol at 20 - 65℃; for 12 - 24h; Stage #2: With sodium hydroxide In methanol; dichloromethane; water pH=9 - 12; |
-
-
81103-11-9
clarithromycin

-
-
127253-06-9
6-methoxyerythromycin A 9-oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate at 80℃; | 90% |
| With hydroxylamine; acetic acid In water; isopropyl alcohol at 40℃; for 120h; Inert atmosphere; | 64% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; Inert atmosphere; | 88% |
| In N,N-dimethyl-formamide at 20℃; for 1.25h; |
-
-
75-44-5
phosgene

-
-
81103-11-9
clarithromycin

-
-
107-18-6
allyl alcohol

-
-
898833-50-6
4''-O-(allyloxycarbonyl)-N-demethylclarithromycin 2',3'-carbamate-11,12-carbonate

| Conditions | Yield |
|---|---|
| Stage #1: phosgene; clarithromycin With pyridine In dichloromethane; toluene at 20℃; for 5h; Stage #2: allyl alcohol In dichloromethane; toluene at 20℃; for 0.5h; Further stages.; | 87% |

-
-
75-77-4
chloro-trimethyl-silane

-
-
81103-11-9
clarithromycin

-
-
130073-49-3
6-O-Methyl-2',4''-O-bis(trimethylsilyl)erythromycin A

| Conditions | Yield |
|---|---|
| With 1-(Trimethylsilyl)imidazole In dichloromethane for 0.5h; Ambient temperature; | 80% |
-
-
994-30-9
triethylsilyl chloride

-
-
81103-11-9
clarithromycin

-
-
1009561-37-8
2',4''-di-TES-6-O-methyl-erythromycin A

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In acetonitrile at 0 - 30℃; Inert atmosphere; | 80% |
-
-
81103-11-9
clarithromycin

-
-
208456-82-0
3-hydroxy-6-O-methyl-10,11-anhydroerythromycin A

| Conditions | Yield |
|---|---|
| Stage #1: clarithromycin With [1,3]-dioxolan-2-one; triethylamine for 44h; Heating / reflux; Stage #2: With hydrogenchloride In ethanol; water at 20℃; for 24h; Stage #3: With potassium hydroxide In ethanol; water pH=10 - 11; | 74% |
| Multi-step reaction with 5 steps 1: hydrogenchloride / water / 2 h / 20 °C 2: dichloromethane / 1 h / 20 °C 3: pyridine / dichloromethane / 9 h / -5 °C 4: potassium tert-butylate; allyl bromide / tetrahydrofuran; dimethyl sulfoxide / 0.5 h / 0 °C 5: methanol / Reflux View Scheme |
-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With water-d2; lithium carbonate; triisopropylsilanethiol In 1-methyl-pyrrolidin-2-one at 20℃; Irradiation; | 70% |

-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| In methanol at 30 - 35℃; for 48h; | 60% |
-
-
81103-11-9
clarithromycin

-
-
622-78-6
Benzyl isothiocyanate

| Conditions | Yield |
|---|---|
| With iodine; sodium hydrogencarbonate; 2-amino-2-hydroxymethyl-1,3-propanediol In acetonitrile for 2h; Irradiation; | 56% |
-
-
81103-11-9
clarithromycin

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran; methanol at 0 - 20℃; for 25h; Inert atmosphere; | 52% |
-
-
81103-11-9
clarithromycin

-
-
124-63-0
methanesulfonyl chloride

-
-
129318-85-0
2',4''-di-O-methanesulfonyl-6-O-methylerythromycin A

| Conditions | Yield |
|---|---|
| With triethylamine In acetone 1.) 0 to 3 deg C, 30 min, 2.) RT, 2 h; | 41.7% |
Clarithromycin Specification
1. Introduction of Clarithromycin
Clarithromycin is one kind of white or almost white crystalline powder or colourless crystalline needles. The Systematic (IUPAC) name of this chemical is (3R,4S,5S,6R,7R,9R,11S,12R,13S,14S)-6-{[(2S,3R,4S,6R) -4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy} -14-ethyl-12,13-dihydroxy-4-{[(2R,4S,5S,6S)-5-hydroxy -4-methoxy-4,6-dimethyloxan-2-yl]oxy}-7 -methoxy-3,5,7,9,11,13-hexamethyl -1-oxacyclotetradecane-2,10-dione. The Classification Code of it is Anti-Bacterial Agents; Anti-Infective Agents; Antibacterial; Drug / Therapeutic Agent; Enzyme Inhibitors; Human Data; Protein synthesis inhibitors.
Clarithromycin belongs to Antibiotics;APIs;Antibiotics for Research and Experimental Use;Biochemistry;Macrolides (Antibiotics for Research and Experimental Use);Intermediates & Fine Chemicals;Pharmaceuticals;API's;1694 Pharmaceuticals&Personal Care Products;A - KAntibiotics;Antibacterial;Antibiotics A to;Antibiotics A-FAntibiotics;Chemical Structure Class;MacrolidesEPA;Neats;Spectrum of Activity. Besides, it should stored in -20°C Freezer.
2. Properties of Clarithromycin
Physical properties about Clarithromycin are:
(1)Index of Refraction: 1.526; (2)Molar Refractivity: 194 cm3; (3)Molar Volume: 631.9 cm3; (4)Density: 1.18 g/cm3; (5)Solubility: Insoluble; (6)Flash Point: 440.9 °C; (7)Melting Point: 217-220 °C; (8)Surface Tension: 48.7 dyne/cm; (9)Enthalpy of Vaporization: 133.36 kJ/mol; (10)Vapour Pressure: 5.06E-30 mmHg at 25 °C; (11)Boiling Point of 6-o-Methoxyerythromycin: 805.5 °C at 760 mmHg.
3. Structure Descriptors of Clarithromycin
(1)Canonical SMILES: CCC1C(C(C(C(=O)C(CC(C(C(C(C(C(=O)O1)C)OC2CC(C(C(O2)C)O)(C)OC)C)OC3C(C(CC
(O3)C)N(C)C)O)(C)OC)C)C)O)(C)O
(2)Isomeric SMILES: CC[C@@H]1[C@@]([C@@H]([C@H](C(=O)[C@@H](C[C@@]([C@@H]([C@H]([C@@H]([C@H](C(=O)O1)C)O[C@H]2C[C@@]([C@H]([C@@H](O2)C)O)(C)OC)C)O[C@H]3[C@@H]([C@H](C[C@H](O3)C)N(C)C)O)(C)OC)C)C)O)(C)O
(3)InChI: InChI=1S/C38H69NO13/c1-15-26-38(10,45)31(42)21(4)28(40)19(2)17-37(9,47-14)33(52-35-29(41)25(39(11)12)16-20(3)48-35)22(5)30(23(6)34(44)50-26)51-27-18-36(8,46-13)32(43)24(7)49-27/h19-27,29-33,35,41-43,45H,15-18H2,1-14H3/t19-,20-,21+,22+,23-,24+,25+,26-,27+,29-,30+,31-,32+,33-,35+,36-,37-,38-/m1/s1
(4)InChIKey: AGOYDEPGAOXOCK-KCBOHYOISA-N
4. Toxicity of Clarithromycin
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD | oral | > 5gm/kg (5000mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BLOOD: HEMORRHAGE | Kiso to Rinsho. Clinical Report. Vol. 22, Pg. 1453, 1988. |
| mouse | LD50 | intraperitoneal | 850mg/kg (850mg/kg) | LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE | Chemotherapy Vol. 36(Suppl, |
| mouse | LD50 | intravenous | 173mg/kg (173mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 22, Pg. 769, 1991. | |
| mouse | LD50 | oral | 1230mg/kg (1230mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Kiso to Rinsho. Clinical Report. Vol. 22, Pg. 1433, 1988. |
| mouse | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | SKIN AND APPENDAGES (SKIN): CORROSIVE: AFTER TOPICAL EXPOSURE | Chemotherapy Vol. 36(Suppl, |
| rat | LD50 | intraperitoneal | 669mg/kg (669mg/kg) | LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SENSE ORGANS AND SPECIAL SENSES: PTOSIS: EYE | Chemotherapy Vol. 36(Suppl, |
| rat | LD50 | oral | 1270mg/kg (1270mg/kg) | BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Kiso to Rinsho. Clinical Report. Vol. 22, Pg. 1433, 1988. |
| rat | LD50 | subcutaneous | > 5gm/kg (5000mg/kg) | SKIN AND APPENDAGES (SKIN): CORROSIVE: AFTER TOPICAL EXPOSURE | Chemotherapy Vol. 36(Suppl, |
| women | TDLo | oral | 30mg/kg/3D-I (30mg/kg) | CARDIAC: CHANGE IN RATE CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP | Annals of Emergency Medicine. Vol. 30, Pg. 542, 1997. |
5. Physical Properties of Clarithromycin
| Physical Property | Value | Units | Temp (deg C) | Source |
|---|---|---|---|---|
| Melting Point | 220 dec | deg C | EXP | |
| pKa Dissociation Constant | 8.99 | (none) | 25 | EXP |
| log P (octanol-water) | 3.16 | (none) | EXP | |
| Water Solubility | 0.342 | mg/L | 25 | EST |
| Vapor Pressure | 8.60E-27 | mm Hg | 25 | EST |
| Henry's Law Constant | 1.73E-29 | atm-m3/mole | 25 | EST |
| Atmospheric OH Rate Constant | 4.78E-10 | cm3/molecule-sec | 25 | EST |
6. Safety information of Clarithromycin
A poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion. Human systemic effects. When heated to decomposition it emits toxic vapors of NOx.
Hazard Codes: Xn,Xi
Risk Statements: 22-36/37/38
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
RTECS: KF4997000
The first aid is as following. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product : Wash mouth out with water, and get medical aid immediately. In addition, it should be stored only in original container, or keep its container tightly closed.
7. Uses of Clarithromycin
The usage of Clarithromycin (CAS NO.81103-11-9) is a semi-synthetic macrolide antibiotic, a derivative of erythromycin. And it is a macrolide antibiotic used to treat upper and lower respiratory tract infections, skin and soft tissue infections. Clarithromycin is a macrolide antibiotic used to treat pharyngitis, tonsillitis, acute maxillary sinusitis, acute bacterial exacerbation of chronic bronchitis, pneumonia, skin and skin structure infections. In addition, it is sometimes used to treat legionellosis, Helicobacter pylori, and lyme disease.
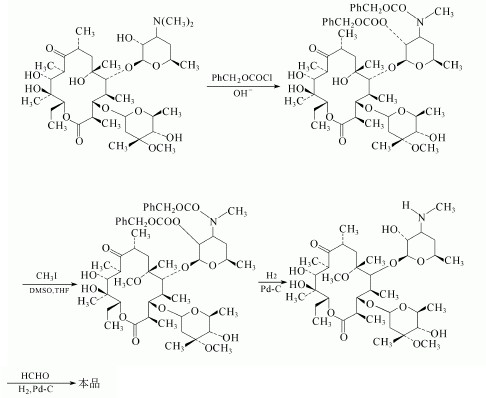
8. Production of Clarithromycin
(1)Use Erythromycin as the raw material and get the methyl off amino by using hydrolysis.
(2)Then get it reaction with Benzyl chloroformate and protect 5-tetrahydropyran ring side-chain hydroxyl and amino.
(3)In the methyl iodide reaction of dimethyl sulfoxide and tetrahydrofuran, carry out six on the methylation of hydroxyl and deprotected by catalytic hydrogenolysis,then reaction with the amino-formaldehyde.
(4)Finally,it is reduced to methyl, named clarithromycin.
The detailed steps are as follows:
Related Products
- Clarithromycin
- 81103-79-9
- 81104-39-4
- 81104-42-9
- 81-10-7
- 81107-97-3
- 81110-73-8
- 81112-09-6
- 81115-43-7
- 81115-45-9
- 81115-67-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View