-
Name
Diethylaminoethanol
- EINECS 202-845-2
- CAS No. 100-37-8
- Article Data52
- CAS DataBase
- Density 0.884 g/cm3
- Solubility soluble in water
- Melting Point -70 °C
- Formula C6H15NO
- Boiling Point 164.8 °C at 760 mmHg
- Molecular Weight 117.191
- Flash Point 48.9 °C
- Transport Information UN 2686 8/PG 2
- Appearance clear to pale yellow liquid
- Safety 25-26-36/37/39-45
- Risk Codes 10-20/21/22-34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 2-diethylaminoethanol;(2-Hydroxyethyl)diethylamine;(Diethylamino)ethanol;2-(Diethylamino)ethanol;2-(Diethylamino)ethyl alcohol;2-(N,N-Diethylamino)ethanol;2-Hydroxytriethylamine;A 22 (amine);DEAE;DEEA;Diethyl(b-hydroxyethyl)amine;Diethylethanolamine;MKS;N,N-Diethyl(2-hydroxyethyl)amine;N,N-Diethyl-2-aminoethanol;N,N-Diethylethanolamine;N,N-Diethylmonoethanolamine;N-(2-Hydroxyethyl)diethylamine;NSC 8759;Pennad 150;b-(Diethylamino)ethanol;
- PSA 23.47000
- LogP 0.32050
Synthetic route

| Conditions | Yield |
|---|---|
| With 3-amino-2-propanol at 70℃; under 4500.45 Torr; for 3h; Pressure; Temperature; | 99.4% |
| With water | |
| water at 85.84 - 94.84℃; under 1500.15 Torr; for 0.116667h; Heating / reflux; | |
| at 76.84 - 112.84℃; under 1500.15 Torr; for 0.133333h; Heating / reflux; |

| Conditions | Yield |
|---|---|
| In ethanol at 60℃; for 6h; Inert atmosphere; | 96% |
-
-
67-56-1
methanol

-
-
84115-05-9
2-(diethylamino)ethyl crotonate

-
A
-
623-43-8
crotonic acid methyl ester

-
B
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 4h; Heating; | A 90% B 82% |

-
-
84115-05-9
2-(diethylamino)ethyl crotonate

-
A
-
623-43-8
crotonic acid methyl ester

-
B
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With methanol; toluene-4-sulfonic acid for 4h; Heating; | A 90% B 82% |
-
-
1120-64-5
2-methyl-4,5-dihydro-1,3-oxazole

-
A
-
110-73-6
2-(Ethylamino)ethanol

-
B
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With platinum on carbon; hydrogen at 25℃; under 7500.75 Torr; for 16h; | A 60% B 19% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-amino-ethanol hydrochloride With potassium carbonate; ethanolamine In acetonitrile Heating; Stage #2: ethyl iodide for 6h; Heating; | 7.8% |
-
-
20570-43-8
carbonic acid ethyl ester-(2-diethylamino-ethyl ester)

-
A
-
100-37-8
2-(Diethylamino)ethanol

-
B
-
105-58-8
Diethyl carbonate

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 40℃; |

| Conditions | Yield |
|---|---|
| at 300℃; |
-
-
22451-37-2
2-diethylamino-ethanol; protonated form

-
-
22719-43-3
2,6-Di-tert-butyl-4-(3,5-di-tert-butyl-4-oxo-cyclohexa-2,5-dienylidenemethyl)-phenol anion

-
A
-
4359-97-1
2,6-di-tert-butyl-4-(3,5-di-tert-butyl-4-hydroxy-benzylidene)-cyclohexa-2,5-dienone

-
B
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; under 682.6 Torr; Equilibrium constant; var. temp., var. pressures; ΔH0, ΔS0; |

-
-
76441-70-8
Diethyl-(2-hydroxy-ethyl)-methoxymethyl-ammonium; bromide

-
A
-
76441-74-2
2-Diethylamino-1-methoxymethoxy-ethan

-
B
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With 2,3-dimercapto-succinic acid at 50℃; |
-
-
107-21-1
ethylene glycol

-
-
109-89-7
diethylamine

-
A
-
150-77-6
N,N,N',N'-Tetraethylethylenediamine

-
B
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With tris(triphenylphosphine)ruthenium(II) chloride at 120℃; for 2h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; | |
| With ruthenium trichloride at 120℃; for 2.5h; Yield given. Yields of byproduct given. Title compound not separated from byproducts; |


| Conditions | Yield |
|---|---|
| With methanol at 25℃; Kinetics; Thermodynamic data; pH 12; ΔH(excit.), ΔS(excit.); |

-
A
-
21351-43-9
4-ethenyl-1-methylpyridinium iodide

-
B
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| In water at 25℃; Rate constant; ionic strength 0.1 mol dm-3; |
-
-
3739-10-4
phenyl-carbamic acid-(2-diethylamino-ethyl ester); hydrochloride

-
A
-
124-38-9
carbon dioxide

-
B
-
62-53-3
aniline

-
C
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 40℃; Rate constant; Thermodynamic data; var. temp., EA; |


| Conditions | Yield |
|---|---|
| With Tris-HCl buffer; human liver carboxylesterase pI 4.5 at 37℃; pH=7.4; Enzyme kinetics; Hydrolysis; | |
| With sodium hydroxide Kinetics; |

-
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With ethanol; sodium |

| Conditions | Yield |
|---|---|
| at 150℃; | |
| With methanol at 45 - 60℃; | |
| at 150℃; |

| Conditions | Yield |
|---|---|
| at 300℃; unter Druck; |


| Conditions | Yield |
|---|---|
| Produkt 2: ein ungesaettigtes Amin; |

| Conditions | Yield |
|---|---|
| at 45 - 60℃; Pr.5: Mono-2-diaethylamino-aethylaether von Tetraaethylenglykol; |


| Conditions | Yield |
|---|---|
| at 5℃; bei der Reduktion; |


| Conditions | Yield |
|---|---|
| zerfaellt beim Erhitzen; |


| Conditions | Yield |
|---|---|
| With water; sodium dodecyl-sulfate; sodium hydroxide at 40℃; Kinetics; Reagent/catalyst; Temperature; |

| Conditions | Yield |
|---|---|
| With potassium tetrachloroplatinate(II); copper(II) choride dihydrate; sulfuric acid; water at 150℃; for 24h; Sealed tube; |
-
-
525-76-8
2-methyl-4-oxo-3,1-benzoxazine

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
82679-12-7
β-(diethylamino)ethyl o-acetamidobenzoate

| Conditions | Yield |
|---|---|
| at 100℃; for 1h; | 100% |
-
-
868765-12-2
2-phenylethynyl-quinoline-3-carbaldehyde

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
1262748-30-0
C24H26N2O2

| Conditions | Yield |
|---|---|
| With triphenylphosphine at 20℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| With silver(l) oxide at 20℃; for 1.5h; Inert atmosphere; regioselective reaction; | 100% |
| With triphenylphosphine at 20℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl-diazodicarboxylate In dichloromethane at 20℃; for 0.5h; Mitsunobu reaction; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile at -20 - 40℃; for 60.5h; | 100% |
| In acetonitrile at 20 - 40℃; for 60.5h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(Diethylamino)ethanol With sodium hydride In tetrahydrofuran at 25 - 40℃; for 0.5h; Stage #2: methyl iodide at 40℃; for 7h; Product distribution / selectivity; | 99.1% |
| Stage #1: 2-(Diethylamino)ethanol With sodium hydride In tetrahydrofuran at 25 - 40℃; for 0.5h; Stage #2: methyl iodide In tetrahydrofuran at 40℃; for 7h; | 99.1% |
-
-
947698-03-5
2-(3,3-dimethylbut-1-ynyl)quinoline-3-carbaldehyde

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
1262748-21-9
C22H30N2O2

| Conditions | Yield |
|---|---|
| With silver(l) oxide at 20℃; for 3h; Inert atmosphere; regioselective reaction; | 99% |
-
-
100-37-8
2-(Diethylamino)ethanol

-
-
1392481-58-1
N,N-diethyl-2-hydroxy-N-(2-(methylsulfonamido)ethyl)ethaneammonium methanesulfonate

| Conditions | Yield |
|---|---|
| In ethanol at 100℃; for 24h; | 99% |
-
-
4774-14-5
2,6-dichloropyrazine

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
1247497-41-1
2-(6-chloropyrazin-2-yloxy)-N,N-diethylethanamine

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 20℃; | 99% |
| Stage #1: 2-(Diethylamino)ethanol With sodium hydride In tetrahydrofuran at 20℃; for 0.166667h; Stage #2: 2,6-dichloropyrazine In tetrahydrofuran at 50℃; for 0.166667h; Microwave irradiation; | 95% |
-
-
56-05-3
2-Amino-4,6-dichloropyrimidine

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
1507138-87-5
4-chloro-6-(2-(diethylamino)ethoxy)pyrimidin-2-amine

| Conditions | Yield |
|---|---|
| With sodium hydride for 0.166667h; Microwave irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 7h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(Diethylamino)ethanol With thionyl chloride In dichloromethane at -10 - 35℃; for 2.5h; Stage #2: With hydrogenchloride | 98.08% |
| With thionyl chloride; chloroform at -5℃; | |
| With thionyl chloride In chloroform |
-
-
100-37-8
2-(Diethylamino)ethanol

-
-
62-23-7
4-nitro-benzoic acid

-
-
13456-39-8
2-(diethylamino)ethyl 4-nitrobenzoate

| Conditions | Yield |
|---|---|
| With formic acid In 5,5-dimethyl-1,3-cyclohexadiene for 24h; Reflux; | 98% |
| With Candida antarctica lipase B immobilised in a macroporous DVB crosslinked polymer (Novozym 435) In cyclohexane at 80℃; for 48h; Enzymatic reaction; |

-
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With sodium | 97.2% |
-
-
56424-15-8
Butyl chloroethynyl sulfide

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
91418-10-9
2-(Butylthiomethylene)-3,3-diethyl-1,3-oxazolidinium chloride

| Conditions | Yield |
|---|---|
| In diethyl ether | 97% |
| In diethyl ether at 20℃; for 3h; | 97% |
-
-
66566-81-2
(iso-butylthio)chloroacetylene

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
97427-23-1
3,3-Diethyl-2-[1-isobutylsulfanyl-meth-(E)-ylidene]-oxazolidin-3-ium; chloride

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 3h; | 97% |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium-containing anion exchanger AV-17-8 In ethanol at 45℃; under 760 Torr; | 97% |

| Conditions | Yield |
|---|---|
| With N,N'-biscyclohexyl-imidazol-2-ylidene; 4 A molecular sieve In tetrahydrofuran at 20℃; for 2h; | 97% |
| With tetraethylammonium bicarbonate at 60℃; for 2h; | 84% |
| With platinum; 1-butyl-3-methylimidazolium Tetrafluoroborate at 20 - 60℃; for 2h; Inert atmosphere; Electrochemical reaction; Green chemistry; | 62% |

| Conditions | Yield |
|---|---|
| Stage #1: homoalylic alcohol; 1,1'-carbonyldiimidazole In dichloromethane at 0 - 20℃; for 3h; Inert atmosphere; Stage #2: 2-(Diethylamino)ethanol In dichloromethane at 20℃; Inert atmosphere; | 97% |

-
-
848592-40-5
5-[6-(3-hydroxy-phenyl)-pyridazin-3-yl]-hexahydro-pyrrolo[3,4-c]pyrrole-2-carboxylic acid tert-butyl ester

-
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In dichloromethane at 0 - 20℃; for 2h; | 96% |
| With di-isopropyl azodicarboxylate; polymer-bound triphenylphosphine In dichloromethane at 0 - 20℃; for 73h; | 54% |
-
-
201230-82-2
carbon monoxide

-
-
39969-57-8
1-bromo-4-butoxybenzene

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
3772-43-8
butoxycaine

| Conditions | Yield |
|---|---|
| With dmap; sodium carbonate; bis(dibenzylideneacetone)-palladium(0); catacxium A In toluene at 100℃; for 18h; Glovebox; Sealed tube; Inert atmosphere; | 96% |
| With dmap; sodium carbonate; bis(dibenzylideneacetone)-palladium(0); catacxium A In toluene at 80℃; Inert atmosphere; Sealed chamber; | 87.4 mg |


| Conditions | Yield |
|---|---|
| Stage #1: aspirin With dicyclohexyl-carbodiimide In chloroform at 5 - 20℃; for 2h; Stage #2: 2-(Diethylamino)ethanol at 20℃; for 3h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: aspirin With dicyclohexyl-carbodiimide In chloroform at 5 - 20℃; for 2h; Stage #2: 2-(Diethylamino)ethanol In chloroform at 20℃; for 3h; | 96% |
| Stage #1: aspirin With dicyclohexyl-carbodiimide In chloroform at 5 - 20℃; for 2h; Stage #2: 2-(Diethylamino)ethanol In chloroform at 20℃; for 3h; | 96% |
-
-
109-89-7
diethylamine

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
74-86-2
acetylene

-
-
3030-43-1
1,5-bis-(N,N-diethylamino)diethylether

| Conditions | Yield |
|---|---|
| With sodium ethanolate In dibutyl ether at 50℃; for 7h; | 95.3% |


| Conditions | Yield |
|---|---|
| With nickel dichloride at 20℃; for 0.5h; Neat (no solvent); | 95% |
| With benzene |
-
-
13057-17-5
bromethyl methyl ether

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
76441-70-8
Diethyl-(2-hydroxy-ethyl)-methoxymethyl-ammonium; bromide

| Conditions | Yield |
|---|---|
| In diethyl ether temperature not higher than 5 deg C; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(Diethylamino)ethanol With sodium hydride In N,N-dimethyl-formamide for 0.5h; Stage #2: 4,7-dichloroquinoline In N,N-dimethyl-formamide for 0.5h; | 95% |
-
-
52386-21-7
2-ethoxy-4,4,6-trimethyl-1,3,2-dioxaborinane

-
-
100-37-8
2-(Diethylamino)ethanol

-
-
13561-17-6
2-diethylaminoethoxy-4,4,6-trimethyl-1,3,2-dioxaborinane

| Conditions | Yield |
|---|---|
| In benzene byproducts: ethanol; amine added to B compd., benzene added, shaken and refluxed for 2 h, moisture excluded; EtOH-benzene distd. azeotropically, solvent evapd. in vac. at room temp., distd.; elem. anal.; | 95% |
Diethylaminoethanol Consensus Reports
Diethylaminoethanol Standards and Recommendations
ACGIH TLV: TWA 10 ppm (skin)
DFG MAK: 5 ppm (24 mg/m3)
DOT Classification: 3; Label: Flammable Liquid
Diethylaminoethanol Analytical Methods
Diethylaminoethanol Specification
The CAS registry number of 2-Diethylaminoethanol is 100-37-8. Its EINECS registry number is 202-845-2. In addition, the molecular formula is C6H15NO. The systematic name is 2-(diethylamino)ethanol. What's more, it is used as pharmaceutical intermediates, softeners, emulsifiers and curing agents.
Physical properties about this chemical are: (1)ACD/LogP: 0.74; (2)ACD/BCF (pH 5.5): 1; (3)ACD/BCF (pH 7.4): 1; (4)ACD/KOC (pH 5.5): 1; (5)ACD/KOC (pH 7.4): 1; (6)#H bond acceptors: 2; (7)#H bond donors: 1; (8)#Freely Rotating Bonds: 5; (9)Polar Surface Area: 23.47 Å2; (10)Index of Refraction: 1.443; (11)Molar Refractivity: 35.1 cm3; (12)Molar Volume: 132.3 cm3; (13)Polarizability: 13.91 ×10-24cm3; (14)Surface Tension: 31.4 dyne/cm; (15)Density: 0.885 g/cm3; (16)Flash Point: 48.9 °C; (17)Enthalpy of Vaporization: 46.73 kJ/mol; (18)Boiling Point: 164.8 °C at 760 mmHg; (19)Vapour Pressure: 0.651 mmHg at 25°C.
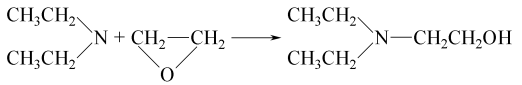
Preparation of 2-Diethylaminoethanol: it can be prepared by diethylamine and epoxyethane at the certain temperature. The equation is as follows:
Uses of 2-Diethylaminoethanol: it can react with isocyanatomethyl-benzene to get N-Benzyl-carbaminsaeure-(2-diethylamino-ethylester). This reaction will need reagent triethylamine and solvent diethyl ether. The yield is about 74% at reaction temperature of 20-30 °C.
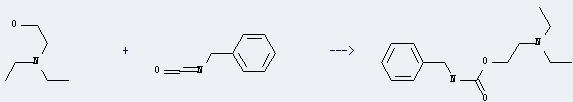
When you are using this chemical, please be cautious about it as the following:
This chemical is flammable and can cause burns. And it is harmful by inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing, gloves and eye/face protection and avoid contact with eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.).
You can still convert the following datas into molecular structure:
(1)SMILES: CCN(CC)CCO
(2)Std.InChI: InChI=1S/C6H15NO/c1-3-7(4-2)5-6-8/h8H,3-6H2,1-2H3
(3)Std.InChIKey: BFSVOASYOCHEOV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | skin | 1mL/kg (1mL/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 26, Pg. 269, 1944. | |
| human | TCLo | inhalation | 200ppm (200ppm) | GASTROINTESTINAL: NAUSEA OR VOMITING | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 216, 1969. |
| mammal (species unspecified) | LDLo | unreported | 1300mg/kg (1300mg/kg) | "Toxicology of Drugs and Chemicals," Deichmann, W.B., New York, Academic Press, Inc., 1969Vol. -, Pg. 216, 1969. | |
| mouse | LC50 | inhalation | 5000mg/m3 (5000mg/m3) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 14(11), Pg. 52, 1970. |
| mouse | LD50 | intramuscular | 416mg/kg (416mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 9, Pg. 31, 1959. | |
| mouse | LD50 | intraperitoneal | 192mg/kg (192mg/kg) | BEHAVIORAL: ATAXIA | Journal of Pharmacology and Experimental Therapeutics. Vol. 94, Pg. 249, 1948. |
| mouse | LD50 | intravenous | 188mg/kg (188mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Arzneimittel-Forschung. Drug Research. Vol. 9, Pg. 31, 1959. |
| mouse | LD50 | subcutaneous | 650mg/kg (650mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Arzneimittel-Forschung. Drug Research. Vol. 9, Pg. 31, 1959. |
| rabbit | LD50 | skin | 1260uL/kg (1.26mL/kg) | Union Carbide Data Sheet. Vol. 6/11/1963, | |
| rat | LCLo | inhalation | 4500mg/m3/4H (4500mg/m3) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS SENSE ORGANS AND SPECIAL SENSES: CONJUNCTIVE IRRITATION: EYE BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 14(11), Pg. 52, 1970. |
| rat | LD50 | intraperitoneal | 1220mg/kg (1220mg/kg) | Toxicology and Applied Pharmacology. Vol. 12, Pg. 486, 1968. | |
| rat | LD50 | oral | 1300mg/kg (1300mg/kg) | Journal of Industrial Hygiene and Toxicology. Vol. 26, Pg. 269, 1944. |
Related Products
- Diethylaminoethanol
- Diethylaminoethanol-p-aminosalicylate
- 1003-78-7
- 100379-00-8
- 10038-16-1
- 10038-40-1
- 1003872-58-9
- 1003876-84-3
- 1003882-41-4
- 1003883-60-0
- 1003887-62-4
- 100-38-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View