-
Name
Malonic acid
- EINECS 205-503-0
- CAS No. 141-82-2
- Article Data303
- CAS DataBase
- Density 1.546 g/cm3
- Solubility water: 1400 g/L (20 °C)
- Melting Point 132-135 °C (dec.)(lit.)
- Formula C3H4O4
- Boiling Point 386.808 °C at 760 mmHg
- Molecular Weight 104.062
- Flash Point 201.905 °C
- Transport Information
- Appearance Crystalline
- Safety 26-36/39-37/39-36
- Risk Codes 20/22-41-36/37/38-22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, Xi
Xi
- Synonyms Dicarboxymethane;Carboxyacetic acid;Malonic acid (Electronical grade);Malonicacid;Malonic acid(medicine grade);Malonic Acid (P516);USAF EK-695;Propanedioic acid Malonic Acid;Propanedioic acid (9CI);Methanedicarboxylic acid;1,3-Propanedioic acid;Malonic acid;propanedioec acid;
- PSA 74.60000
- LogP -0.45430
Synthetic route

| Conditions | Yield |
|---|---|
| With C4H11FeMo6NO24(3-)*3C16H36N(1+); water; oxygen; sodium carbonate at 50℃; under 760.051 Torr; for 8h; Green chemistry; | 99% |
| With 4H3N*4H(1+)*CuMo6O18(OH)6(4-); water; oxygen; sodium carbonate at 50℃; under 760.051 Torr; for 12h; | 93% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | 97% |
| With sodium hydroxide elektrochemische Oxydation, am besten an einer Nickelanode; |

| Conditions | Yield |
|---|---|
| With water at 70 - 80℃; | 97% |
| With water; sodium hydroxide In methanol at 80℃; for 1h; |

| Conditions | Yield |
|---|---|
| With acetic acid In water at 70 - 80℃; Reagent/catalyst; | 95% |
| With hydrogen ethyl malonate; sulfuric acid at 70℃; under 175 Torr; Abdestillieren des entstehenden Aethanols; | |
| With malonic acid; sulfuric acid at 70℃; under 175 Torr; Abdestillieren des entstehenden Aethanols; |
-
-
3815-86-9
malonic acid dihydrazide

-
-
75-15-0
carbon disulfide

-
A
-
141-82-2
malonic acid

-
B
-
1072-71-5
2,5-Dimercapto-1,3,4-thiadiazole

| Conditions | Yield |
|---|---|
| Stage #1: malonic acid dihydrazide; carbon disulfide With potassium hydroxide In ethanol for 3h; Rearrangement; cyclization; Heating; Stage #2: With hydrogenchloride In ethanol Hydrolysis; ring cleavage; | A n/a B 90% |


| Conditions | Yield |
|---|---|
| With niobium(V) oxide; water In neat (no solvent) for 30h; Reflux; Inert atmosphere; | 82% |
-
-
108-59-8
malonic acid dimethyl ester

-
A
-
141-82-2
malonic acid

-
B
-
16695-14-0
Malonic acid monomethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: malonic acid dimethyl ester With potassium hydroxide; water In acetonitrile at 0℃; for 1h; Stage #2: With hydrogenchloride; water In acetonitrile Product distribution / selectivity; | A n/a B 81% |
| Stage #1: malonic acid dimethyl ester With water; potassium hydroxide at 0℃; for 1.5h; Stage #2: Acidic aq. solution; | A 9.8% B 76% |

| Conditions | Yield |
|---|---|
| Stage #1: diethyl malonate With water; potassium hydroxide In tetrahydrofuran at 0℃; for 0.5h; Stage #2: In tetrahydrofuran; water Acidic conditions; | A 8.3% B 76.9% |
| Stage #1: diethyl malonate With water; potassium hydroxide at 0℃; for 6h; Stage #2: Acidic aq. solution; | A 36.6% B 35.6% |

| Conditions | Yield |
|---|---|
| With phosphotungstic acid; dihydrogen peroxide; cetylpyridinium bromide In water at 85℃; for 5h; Concentration; Green chemistry; | A 49.8% B 60.7% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at 20℃; for 18h; | 60% |

| Conditions | Yield |
|---|---|
| With iron disulphate; dihydrogen peroxide at 2℃; | A 16% B 12% C 58% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium permanganate at 0℃; for 0.05h; | A 8.5% B 57% |
| With potassium hydroxide; potassium permanganate In water at 0℃; for 0.05h; Product distribution; Mechanism; | A 8.5% B 57% |
-
-
7270-63-5
5-diazo-2,2-dimethyl-1,3-dioxane-4,6-dion

-
A
-
68695-08-9
6,6-dimethyl-4,8-dioxo-5,7-dioxa-1,2-diazaspiro<2.5>oct-1-ene

-
B
-
2033-24-1
cycl-isopropylidene malonate

-
C
-
80-69-3
tartronic acid

-
D
-
141-82-2
malonic acid

-
E
-
62609-78-3
2,2-dimethyl-5-oxo-1,3-dioxolane-4-carboxylic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water for 3.5h; Product distribution; Irradiation; | A 14% B 5% C 12% D 6% E 56% |

-
-
7597-56-0
ethyl malonamate

-
A
-
60-35-5
acetamide

-
B
-
141-82-2
malonic acid

-
C
-
1071-46-1
hydrogen ethyl malonate

-
D
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With phthalic anhydride at 240 - 250℃; under 3040 Torr; for 1h; Hydrolysis; | A n/a B n/a C 53% D n/a |

-
-
615-94-1
2,5-dihydroxy-1,4-benzoquinone

-
A
-
141-82-2
malonic acid

-
B
-
124-38-9
carbon dioxide

-
C
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water at 39.99℃; for 0.333333h; Kinetics; Thermodynamic data; Mechanism; Temperature; Time; | A 52.1% B n/a C n/a |

-
-
67-56-1
methanol

-
-
2033-24-1
cycl-isopropylidene malonate

-
-
485-47-2
indan-1,2,3-trione hydrate

-
A
-
141-82-2
malonic acid

| Conditions | Yield |
|---|---|
| Stage #1: cycl-isopropylidene malonate; indan-1,2,3-trione hydrate With montmorillonite K10 clay In methanol at 20℃; for 0.0833333h; Stage #2: methanol In ethyl acetate | A n/a B 40% |


| Conditions | Yield |
|---|---|
| Sonication; | A 40% B 30% C 13% |

-
-
1165952-92-0
cyclohexa-1,4-diene

-
-
141-82-2
malonic acid

| Conditions | Yield |
|---|---|
| With ozone In methanol for 1h; Product distribution; Heating; followed by reaction with aq. H2O2 / HCOOH; | 30% |
| With formic acid; dihydrogen peroxide; ozone 1.) methanol, -40 deg C, 2.) reflux, 1 h; Yield given. Multistep reaction; |
-
-
50-99-7
D-glucose

-
A
-
64-18-6
formic acid

-
B
-
79-14-1
glycolic Acid

-
C
-
79-33-4
L-Lactic acid

-
D
-
141-82-2
malonic acid

-
E
-
110-15-6
succinic acid

-
F
-
144-62-7
oxalic acid

-
G
-
64-19-7
acetic acid

-
H
-
802294-64-0
propionic acid

-
I
-
127-17-3
2-oxo-propionic acid

-
J
-
110-16-7
maleic acid

| Conditions | Yield |
|---|---|
| With sodium silicate; water at 300℃; under 64356.4 Torr; for 0.0166667h; Reagent/catalyst; Sealed tube; | A n/a B n/a C 30% D n/a E n/a F n/a G n/a H n/a I n/a J n/a |

-
-
2033-24-1
cycl-isopropylidene malonate

-
-
64-17-5
ethanol

-
-
485-47-2
indan-1,2,3-trione hydrate

-
A
-
141-82-2
malonic acid

-
B
-
1176972-62-5
ethyl (2'-hydroxyindane-1',3'-dione-2'-yl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: cycl-isopropylidene malonate; indan-1,2,3-trione hydrate With montmorillonite K10 clay In ethanol at 20℃; for 0.0833333h; Stage #2: ethanol In ethyl acetate | A n/a B 25% |

-
-
110-83-8
cyclohexene

-
A
-
110-62-3
pentanal

-
B
-
692-29-5
Succinic semialdehyde

-
C
-
141-82-2
malonic acid

-
D
-
144-62-7
oxalic acid

| Conditions | Yield |
|---|---|
| With ozone at 24.85℃; Product distribution; in dark; | A 17.05% B 6.9% C 6.88% D 6.16% |


| Conditions | Yield |
|---|---|
| With water for 0.833333h; argon plasma-jet; | 13% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
21577-50-4
mucobromic acid

-
-
141-82-2
malonic acid

| Conditions | Yield |
|---|---|
| With barium dihydroxide beim Kochen; |
-
-
187737-37-7
propene

-
-
141-82-2
malonic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate |

| Conditions | Yield |
|---|---|
| With water | |
| With water In diethyl ether C3O2 dissolved in ether;; | |
| With water; hydrogenchloride In water | |
| With water In water during hours;; | >99 |
| With water In water |

| Conditions | Yield |
|---|---|
| With oxygen in Gegenwart einer dialysierten Enzymloesung aus Schweineherz und von MnCl2; |

-
-
141-82-2
malonic acid

| Conditions | Yield |
|---|---|
| With nitric acid |

| Conditions | Yield |
|---|---|
| With acetic acid at 20℃; for 1h; | 100% |
| With acetic acid |
-
-
13679-70-4
5-methylthiophene-2-carboxaldehyde

-
-
141-82-2
malonic acid

-
-
70329-36-1
(E)-3-(5-methyl-2-thienyl)-2-propenoic acid

| Conditions | Yield |
|---|---|
| With piperidine; pyridine Heating; | 100% |
| With piperidine; pyridine Reflux; | 66% |
| With piperidine; pyridine Heating / reflux; | 66% |

| Conditions | Yield |
|---|---|
| With boron trifluoride at 65℃; for 0.333333h; | 100% |
| zirconium(IV) oxide; molybdenum at 84.85℃; for 6h; Esterification; | 95% |
| iodine for 15h; Heating; | 94% |
-
-
141-82-2
malonic acid

-
-
15356-60-2
(1S,2R,5S)-(+)-menthol

-
-
73636-64-3, 131348-66-8, 141611-48-5
Malonsaeure-bis<(1R)-menthyl>ester

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In diethyl ether for 2h; Ambient temperature; | 100% |
| at 100℃; Einleiten von HCl; |

| Conditions | Yield |
|---|---|
| With pyridine In ethanol for 1.75h; Knoevenagel condensation; Inert atmosphere; Reflux; | 100% |
| With ammonium acetate for 0.0666667h; Irradiation; | 95% |
| Stage #1: malonic acid; 4-nitrobenzaldehdye With piperidine; pyridine Knoevenagel-Doebner reaction; Reflux; Stage #2: With hydrogenchloride In water Cooling with ice; optical yield given as %de; | 95% |

| Conditions | Yield |
|---|---|
| With piperidine; pyridine | 100% |
| With piperidine; pyridine at 115℃; for 3h; Knoevenagel Condensation; | 93% |
| With pyridine for 1h; Heating; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: malonic acid; cyclohexanecarbaldehyde With piperidine; pyridine at 20 - 80℃; for 6h; Doebner reaction; Stage #2: With hydrogenchloride In water Cooling with ice; | 100% |
| With piperidine; pyridine | 97% |
| With piperidine; pyridine |
-
-
455-19-6
4-Trifluoromethylbenzaldehyde

-
-
141-82-2
malonic acid

-
-
16642-92-5, 87212-84-8, 2062-26-2
4-(trifluoromethyl)cinnamic acid

| Conditions | Yield |
|---|---|
| With pyridine; aniline In toluene for 18h; Doebner Modification; Reflux; | 100% |
| With pyridine; ammonium acetate at 85℃; for 5h; Darkness; | 65.36% |
-
-
99845-89-3
3-formyl-5,5'-dimethyl-2,2'-bithiophene

-
-
141-82-2
malonic acid

| Conditions | Yield |
|---|---|
| With piperidine; pyridine for 2h; Heating; | 100% |
-
-
141-82-2
malonic acid

-
-
24973-22-6
3-methoxy-4-methylbenzaldehyde

-
-
132980-20-2
3-(3-Methoxy-4-methylphenyl)-2-propenoic acid

| Conditions | Yield |
|---|---|
| Stage #1: malonic acid; 3-methoxy-4-methylbenzaldehyde With piperidine; pyridine for 2.5h; Heating / reflux; Stage #2: With hydrogenchloride In water | 100% |
| In piperidine; pyridine for 2.5h; Heating / reflux; | 100% |
| With piperidine; pyridine for 5h; Heating; | 99.7% |
-
-
141-82-2
malonic acid

-
-
93656-82-7
1-methoxypyrene-8-carboxaldehyde

| Conditions | Yield |
|---|---|
| With piperidine; pyridine 1.) from 80 deg C to 85 deg C, 30 min, 2.) reflux, 4 h; | 100% |

| Conditions | Yield |
|---|---|
| copper(I) oxide In dimethyl sulfoxide at 110 - 120℃; for 1.5h; Product distribution / selectivity; | 100% |
| copper(I) oxide In N,N-dimethyl-formamide at 110 - 120℃; for 1.5h; Product distribution / selectivity; | 100% |
| In neat (no solvent) at 141℃; Kinetics; Thermodynamic data; ΔH(excit.), ΔS(excit.), ΔF(excit.); further solvent; |

| Conditions | Yield |
|---|---|
| With pyridine; glycine at 80℃; for 3h; Catalytic behavior; Reagent/catalyst; Sealed tube; | 100% |
| With triton-B adsorbed on flyash In neat (no solvent) for 0.0152778h; Microwave irradiation; Green chemistry; | 96% |
| Stage #1: malonic acid With triethylamine In toluene Knoevenagel Condensation; Stage #2: 4-hydroxy-benzaldehyde With piperidine In toluene for 2h; Knoevenagel Condensation; Reflux; | 88% |
-
-
141-82-2
malonic acid

-
-
1571-08-0
methyl 4-formylbenzoate

-
-
19473-96-2
4-(2-carboxyvinyl)benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With piperidine; pyridine at 120℃; for 12h; | 100% |
| With piperidine In pyridine at 100 - 120℃; for 19h; | 99.39% |
| With piperidine; pyridine at 80 - 100℃; for 4h; | 91.6% |
| With piperidine; pyridine at 110℃; for 2h; | 75% |
| With piperidine; pyridine for 5h; Reflux; | 36% |
-
-
141-82-2
malonic acid

-
-
183145-78-0
[tert-Butoxycarbonyl-(2-formyl-phenyl)-amino]-acetic acid methyl ester

-
-
183145-79-1
(E)-3-[2-(tert-Butoxycarbonyl-methoxycarbonylmethyl-amino)-phenyl]-acrylic acid

| Conditions | Yield |
|---|---|
| With piperidine; pyridine for 1h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With piperidine; pyridine for 2.5h; Heating / reflux; Neat (no solvent); | 100% |
| With piperidine; pyridine for 2.5h; Heating / reflux; | 100% |
| With piperidine; pyridine at 100℃; Condensation; |

| Conditions | Yield |
|---|---|
| With pyridine; aniline In toluene for 18h; Doebner Modification; Reflux; | 100% |
| With triton-B adsorbed on flyash In neat (no solvent) for 0.0125h; Microwave irradiation; Green chemistry; | 96% |
| With 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 100 - 110℃; for 1.25h; Knoevenagel-Doebner-Stobbe Reaction; | 95% |
-
-
141-82-2
malonic acid

-
-
59565-09-2
2-(vinyloxy)ethyl isothiocyanate

| Conditions | Yield |
|---|---|
| at 65 - 100℃; for 0.166667h; | 100% |
-
-
18791-75-8
4-Bromothiophen-2-aldehyde

-
-
141-82-2
malonic acid

-
-
103686-16-4
trans-3-(4-bromothiophen-2-yl)propenoic acid

| Conditions | Yield |
|---|---|
| With piperidine; pyridine at 100℃; | 100% |
| Stage #1: 4-Bromothiophen-2-aldehyde; malonic acid With piperidine; pyridine at 100℃; for 24h; Stage #2: With hydrogenchloride In water pH=3; | 100% |
| piperidine In pyridine at 100℃; for 22h; Knoevenagel reaction; | 96% |
-
-
141-82-2
malonic acid

-
-
24973-22-6
3-methoxy-4-methylbenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: malonic acid; 3-methoxy-4-methylbenzaldehyde With piperidine; pyridine for 11h; Heating / reflux; Stage #2: With hydrogenchloride In water | 100% |
-
-
141-82-2
malonic acid

-
-
65253-04-5
(-)-8-phenylmenthol

-
-
75863-05-7
Bis<(1S,2R,4R)-2-methyl-5-(1-methyl-1-phenylethyl)cyclohexyl>malonate

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In diethyl ether at 20℃; for 0.5h; | 100% |
-
-
141-82-2
malonic acid

-
-
52178-50-4
Methyl 3-formylbenzoate

-
-
98116-12-2
(E)-3-(3-methoxycarbonylphenyl)acrylic acid

| Conditions | Yield |
|---|---|
| With PYRIMIDINE In N,N-dimethyl-formamide at 90℃; for 10h; Inert atmosphere; | 100% |
| With piperidine; pyridine Knoevenagel Condensation; Reflux; | 75% |
| With piperidine; pyridine at 80℃; for 4h; | 72% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 0.5h; | 100% |
| In tetrahydrofuran Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile Inert atmosphere; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: (E)-3-[5,10,15,20-tetrakis(3,5-dimethylphenyl)porphyrin-2-yl]-2-propenal; malonic acid With ammonium acetate; acetic acid at 70℃; for 3h; Stage #2: zinc diacetate at 70℃; for 0.25h; | 100% |


| Conditions | Yield |
|---|---|
| In water; acetone for 0.166667h; | 100% |
-
-
141-82-2
malonic acid

-
-
1262518-41-1
3-[4-(1-ethoxycarbonylpiperidin-4-ylidene)-9,10-dihydro-4H-1-thiabenzo[f]azulen-2-yl]acrylic acid

| Conditions | Yield |
|---|---|
| With piperidine; hydrogenchloride In pyridine Reflux; | 100% |
-
-
141-82-2
malonic acid

-
-
139978-82-8
methyl 2,3-O-isopropylidene-α-D-mannopyranoside-4,6-cyclic sulfate

| Conditions | Yield |
|---|---|
| Stage #1: malonic acid; methyl-2,3-O-isopropylidene-4,6-cyclic sulfate-α-D-mannopyranoside With sodium hydride In N,N-dimethyl-formamide Stage #2: In tetrahydrofuran; methanol regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| at 100℃; | 100% |
| at 20℃; for 24h; | |
| at 50 - 120℃; | |
| at 80℃; |

| Conditions | Yield |
|---|---|
| With piperidine; pyridine at 95℃; | 100% |
Malonic acid Chemical Properties
Molecular Structure of Malonic acid (CAS NO. 141-82-2):
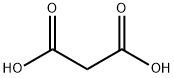
IUPAC Name: Propanedioic acid
Molecular Formula: C3H4O4
Molecular Weight: 104.061460 g/mol
XLogP3: -0.8
H-Bond Donor: 2
H-Bond Acceptor: 4
Canonical SMILES: C(C(=O)O)C(=O)O
InChI: InChI=1S/C3H4O4/c4-2(5)1-3(6)7/h1H2,(H,4,5)(H,6,7)
InChIKey: OFOBLEOULBTSOW-UHFFFAOYSA-N
Index of Refraction: 1.478
Molar Refractivity: 19.07 cm3
Molar Volume: 67.3 cm3
Surface Tension: 70.5 dyne/cm
Density: 1.546 g/cm3
Flash Point: 201.9 °C
Enthalpy of Vaporization: 69.8 kJ/mol
Boiling Point: 386.8 °C at 760 mmHg
Vapour Pressure: 4.66E-07 mmHg at 25 °C
Melting Point: 132-135 °C (dec.)(lit.)
Water Solubility: 1400 g/L (20 ºC)
Storage Temp.: Store at RT.
BRN: 1751370
EINECS: 205-503-0
Product Categories: Intermediates;Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts;alpha,omega-Alkanedicarboxylic Acids;alpha,omega-Bifunctional Alkanes;Monofunctional & alpha,omega-Bifunctional Alkanes
Malonic acid Uses
Malonic acid (141-82-2) is used as building block in chemical synthesis. frequently used as an enolate in Knoevenagel condensations or condensed with acetone to form Meldrum's acid. Its esters are also used for the -CH2COOH synthon in the malonic ester synthesis.
Malonic acid Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 300mg/kg (300mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| mouse | LD50 | oral | 4gm/kg (4000mg/kg) | Biochemical Journal. Vol. 34, Pg. 1196, 1940. | |
| rabbit | LDLo | skin | 10gm/kg (10000mg/kg) | BIOFAX Industrial Bio-Test Laboratories, Inc., Data Sheets. Vol. 22-3/1971, | |
| rat | LC50 | inhalation | > 8900mg/m3/1H (8900mg/m3) | BIOFAX Industrial Bio-Test Laboratories, Inc., Data Sheets. Vol. 22-3/1971, | |
| rat | LD50 | intraperitoneal | 1500mg/kg (1500mg/kg) | "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2C, Pg. 4937, 1982. | |
| rat | LD50 | oral | 1310mg/kg (1310mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA LUNGS, THORAX, OR RESPIRATION: CYANOSIS | BIOFAX Industrial Bio-Test Laboratories, Inc., Data Sheets. Vol. 22-3/1971, |
Malonic acid Safety Profile
Safety Information of Malonic acid (CAS NO. 141-82-2):
Hazard Codes: Xn,Xi 
Risk Statements: 20/22-41-36/37/38-22
R20/22: Harmful by inhalation and if swallowed
R41: Risk of serious damage to the eyes
R36/37/38: Irritating to eyes, respiratory system and skin
R22:Harmful if swallowed
Safety Statements: 26-36/39-37/39-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S37/39:Wear suitable gloves and eye/face protection
S36/39:Wear suitable protective clothing and eye/face protection
S36:Wear suitable protective clothing
RIDADR: 3261
WGK Germany: 1
RTECS: OO0175000
PackingGroup: III
HS Code: 29171910
When heated to decomposition it emits acrid smoke and irritating vapors.
Malonic acid Specification
Malonic acid with cas registry number of 141-82-2 is is a carboxylic acid, also called for AI3-15375 ; Carboxyacetic acid ; Dicarboxymethane ; Kyselina malonova ; Kyselina malonova [Czech] ; MALONIC ACID ; Methanedicarboxylic acid ; NSC 8124 ; Propanedioic acid ; USAF EK-695 . It can be prepared in this way: Sodium carbonate generates the sodium salt which is then reacted with sodium cyanide to provide the cyano acetic acid salt via a nucleophilic substitution. The nitrile group can be hydrolyzed with sodium hydroxide to sodium malonate and acidification affords malonic acid.
Related Products
- Malonic acid
- Malonic acid bis(2,4,6-trichlorophenyl) ester
- Malonic acid disodium salt monohydrate
- Malonic acid, (trifluoromethyl)-, dimethyl ester
- Malonic acid, ethylmethyl-
- Malonic acid, ethylphenyl-, 2-(diethylamino)ethyl ethyl ester
- Malonic acid,(3-isothiazolylmethyl)methyl-, diethyl ester (7CI,8CI)
- Malonic dihydrazide
- 14182-37-7
- 141-84-4
- 141849-62-9
- 141850-54-6
- 141852-65-5
- 141854-25-3
- 141-85-5
- 14185-99-0
- 14-18-6
- 14186-50-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View