-
Name
METHYL 2-OCTYNOATE
- EINECS 203-836-6
- CAS No. 111-12-6
- Article Data17
- CAS DataBase
- Density 0.92 g/mL at 25 °C(lit.)
- Solubility Almost insoluble in water
- Melting Point
- Formula C9H14 O2
- Boiling Point 217-220 °C(lit.)
- Molecular Weight 154.209
- Flash Point 192 °F
- Transport Information
- Appearance colorless liquid
- Safety Moderately toxic by ingestion and skin contact. A moderate skin and eye irritant. A combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
- Risk Codes 22-38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Folione;Methyl 2-octynate; Methyl 2-octynoate; Methyl pentylacetylenecarboxylate; NSC72098; Vert de violette, artificial
- PSA 26.30000
- LogP 1.74310
Synthetic route

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran 1.) -78 deg C, 30 min, 2.) -78 deg C to r.t., 15 min; | 97% |

| Conditions | Yield |
|---|---|
| In diethyl ether | 95% |
| In diethyl ether | 10.03 g |

| Conditions | Yield |
|---|---|
| With cesium fluoride In N,N-dimethyl-formamide at 10 - 15℃; for 24h; | 90% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; copper(ll) bromide; palladium(II) bromide at 20℃; under 760 Torr; for 2h; | 83% |
| With oxygen; sodium acetate; triphenylphosphine; palladium dichloride In N,N-dimethyl-formamide at 20℃; for 48h; | 75% |
| With sodium acetate; copper dichloride; palladium dichloride under 760 Torr; for 2h; Ambient temperature; | 74 % Chromat. |

-
-
93296-09-4
4-methoxyphenylzinc chloride

-
-
176246-52-9
methyl (E)-2,3-dibromo-2-octenoate

-
A
-
111-12-6
methyl 2-octynoate

-
B
-
2132-80-1
4,4'-Dimethoxybiphenyl

| Conditions | Yield |
|---|---|
| With palladium diacetate; triphenyl-arsane In tetrahydrofuran at 20℃; for 21.5h; Further byproducts given; | A n/a B n/a C 61% D n/a |

-
-
68113-72-4
1-octynylzinc chloride

-
-
176246-52-9
methyl (E)-2,3-dibromo-2-octenoate

-
A
-
111-12-6
methyl 2-octynoate

| Conditions | Yield |
|---|---|
| With palladium diacetate; triphenyl-arsane In tetrahydrofuran at 20℃; for 23h; | A n/a B 57% C n/a |

-
-
133472-27-2
4-fluorophenylzinc chloride

-
-
176246-52-9
methyl (E)-2,3-dibromo-2-octenoate

-
A
-
111-12-6
methyl 2-octynoate

-
B
-
398-23-2
4,4'-difluorobiphenyl

| Conditions | Yield |
|---|---|
| With palladium diacetate; triphenyl-arsane In tetrahydrofuran at 20℃; for 24h; | A n/a B n/a C 56% D n/a |


-
-
176246-52-9
methyl (E)-2,3-dibromo-2-octenoate

-
A
-
111-12-6
methyl 2-octynoate

-
B
-
121169-22-0
2,2'-bis(methoxymethoxy)-1,1'-biphenyl

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 20℃; for 71h; | A n/a B n/a C 43% D n/a |


| Conditions | Yield |
|---|---|
| With lead(IV) acetate for 0.25h; | 38% |

| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With hydrogenchloride | 8.9 g |
-
-
63125-02-0
Methyl-2-hexanoyl-2-triphenylphosphoranylidenacetat

-
-
111-12-6
methyl 2-octynoate

| Conditions | Yield |
|---|---|
| at 220 - 250℃; |

| Conditions | Yield |
|---|---|
| With sodium carbonate at -20℃; for 0.5h; Hydrolysis; |
-
-
194856-56-9
Oct-2-ynylsulfanyl-benzene

-
-
111-12-6
methyl 2-octynoate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine; sulfuryl chloride / CCl4 / 0.5 h / 0 °C 2: sodium carbonate / 0.5 h / -20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: benzene 2: 220 - 250 °C View Scheme |

| Conditions | Yield |
|---|---|
| With formic acid; (1,2-dimethoxyethane)dichloronickel(II); zinc; bis(2-diphenylphosphinoethyl)phenylphosphine In 1,4-dioxane at 120℃; for 16h; Sealed tube; | 100% |
-
-
111-12-6
methyl 2-octynoate

-
-
176246-52-9
methyl (E)-2,3-dibromo-2-octenoate

| Conditions | Yield |
|---|---|
| With pyridinium hydrobromide perbromide In dichloromethane for 192h; | 98% |
-
-
111-12-6
methyl 2-octynoate

-
-
68854-59-1
2-cis octenoic acid methyl ester

| Conditions | Yield |
|---|---|
| With ammonium formate In N,N-dimethyl-formamide at 80℃; Green chemistry; diastereoselective reaction; | 96% |
| Stage #1: methyl 2-octynoate With tris-(dibenzylideneacetone)dipalladium(0); 1,4-di(diphenylphosphino)-butane In 1,4-dioxane at 20℃; for 0.25h; Inert atmosphere; Stage #2: With formic acid In 1,4-dioxane at 80℃; for 3h; Inert atmosphere; optical yield given as %ee; stereoselective reaction; | 90% |
| With formic acid; nickel dibromide; zinc In 1,4-dioxane at 120℃; for 16h; Sealed tube; stereoselective reaction; | 82% |
| With hydrogen; Lindlar's catalyst | |
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; formic acid; tributylphosphine; triethylamine In tetrahydrofuran at 40℃; for 2h; | 95 % Chromat. |

| Conditions | Yield |
|---|---|
| With palladium diacetate In trifluoroacetic acid at 20℃; for 77h; | 96% |

| Conditions | Yield |
|---|---|
| With copper diacetate In methanol at 28℃; for 2.5h; Inert atmosphere; stereoselective reaction; | 96% |
| With methanol; copper (I) acetate at 28℃; for 24h; Inert atmosphere; stereoselective reaction; | 94% |
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane In tetrahydrofuran; acetonitrile at 80℃; for 12h; Sealed tube; Inert atmosphere; stereoselective reaction; | 93% |
-
-
111-12-6
methyl 2-octynoate

-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
176246-71-2
methyl (E)-3-(4-methoxyphenyl)-2-octenoate

| Conditions | Yield |
|---|---|
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane In tetrahydrofuran; acetonitrile at 80℃; for 12h; Sealed tube; Inert atmosphere; stereoselective reaction; | 96% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; for 16h; Sealed tube; | 96% |

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 10h; Inert atmosphere; stereoselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 10h; Inert atmosphere; stereoselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 4h; Inert atmosphere; stereoselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 4h; Inert atmosphere; stereoselective reaction; | 94% |
-
-
111-12-6
methyl 2-octynoate

-
-
5467-74-3
4-Bromophenylboronic acid

-
-
1126701-07-2
methyl (E)-3-(4-bromophenyl)-2-octenoate

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 24h; Inert atmosphere; stereoselective reaction; | 94% |
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane In tetrahydrofuran; acetonitrile at 80℃; for 12h; Sealed tube; Inert atmosphere; stereoselective reaction; | 93% |
-
-
111-12-6
methyl 2-octynoate

-
-
13331-27-6
m-nitrobenzene boronic acid

-
-
1126701-04-9
methyl (E)-3-(3-nitrophenyl)-2-octenoate

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 5h; Inert atmosphere; stereoselective reaction; | 94% |
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane In tetrahydrofuran; acetonitrile at 80℃; for 12h; Sealed tube; Inert atmosphere; stereoselective reaction; | 67% |
-
-
111-12-6
methyl 2-octynoate

-
-
87199-16-4
3-Formylphenylboronic acid

-
-
1126701-02-7
methyl (E)-3-(3-formylphenyl)-2-octenoate

| Conditions | Yield |
|---|---|
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane In tetrahydrofuran; acetonitrile at 80℃; for 12h; Sealed tube; Inert atmosphere; stereoselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2-octynoate With tris-(dibenzylideneacetone)dipalladium(0); 1,4-di(diphenylphosphino)-butane In 1,4-dioxane; water at 20℃; for 0.25h; Inert atmosphere; Stage #2: With formic acid In 1,4-dioxane at 80℃; for 10h; Inert atmosphere; optical yield given as %ee; stereoselective reaction; | 93% |
| Stage #1: methyl 2-octynoate With Triethoxysilane; tris(acetonitrile)pentamethylcyclopentadienylruthenium(II) hexafluorophosphate In dichloromethane at 20℃; for 0.5h; Stage #2: With copper(l) iodide; tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 20h; Further stages.; | 83% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-dihydronaphthalene-1,4-epoxide With zinc; dibromo[1,2-bis(diphenylphosphino)ethane]nickel(II) In acetonitrile at 20℃; Stage #2: methyl 2-octynoate With water In acetonitrile at 20℃; for 16h; Further stages.; | 93% |

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 5h; Inert atmosphere; stereoselective reaction; | 93% |
-
-
292638-84-7
styrene

-
-
111-12-6
methyl 2-octynoate

-
-
1392484-85-3
(E)-3-(1-chlorohexylidene)-dihydro-5-phenylfuran-2(3H)-one

| Conditions | Yield |
|---|---|
| With copper(II) choride dihydrate; oxygen; palladium dichloride In acetonitrile; benzene at 100℃; under 760.051 Torr; for 12h; | 93% |
-
-
111-12-6
methyl 2-octynoate

-
-
72824-04-5
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
1379654-29-1
C12H20O2

| Conditions | Yield |
|---|---|
| With copper diacetate In methanol at -78 - 25℃; for 3h; Inert atmosphere; stereoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: methyl 2-octynoate With tris-(dibenzylideneacetone)dipalladium(0); tricyclohexylphosphine In 1,4-dioxane at 20℃; for 0.25h; Inert atmosphere; Stage #2: With formic acid In 1,4-dioxane at 80℃; Inert atmosphere; chemoselective reaction; | 91% |
| With o-phenylenebis(diphenylphosphine); copper diacetate; poly(methylhydrosiloxane) In toluene; tert-butyl alcohol at 20℃; for 3h; | 90% |
| With magnesium In methanol Ambient temperature; reacted until the Mg had dissolved; | 76% |

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 6h; Inert atmosphere; stereoselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 3h; Inert atmosphere; stereoselective reaction; | 91% |
| With methanol; copper (I) acetate at 28℃; for 3h; stereoselective reaction; | 91% |
-
-
111-12-6
methyl 2-octynoate

-
-
10365-98-7
3-methoxyphenylboronic acid

-
-
1126701-00-5
methyl (E)-3-(3-methoxyphenyl)-2-octenoate

| Conditions | Yield |
|---|---|
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane In tetrahydrofuran; acetonitrile at 80℃; for 12h; Sealed tube; Inert atmosphere; stereoselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 16h; Sealed tube; | 91% |

| Conditions | Yield |
|---|---|
| With copper (I) acetate In methanol at 28℃; for 24h; Inert atmosphere; stereoselective reaction; | 90% |

| Conditions | Yield |
|---|---|
| With cobalt acetylacetonate; 1,2-bis-(diphenylphosphino)ethane In tetrahydrofuran; acetonitrile at 80℃; for 12h; Sealed tube; Inert atmosphere; stereoselective reaction; | 90% |
-
-
111-12-6
methyl 2-octynoate

-
-
107-18-6
allyl alcohol

-
-
1446623-66-0
(E)-methyl2-allyl-3-bromooct-2-enoate

| Conditions | Yield |
|---|---|
| With copper(ll) bromide; palladium dichloride In tetrahydrofuran at 25℃; for 12h; Solvent; Reagent/catalyst; regioselective reaction; | 90% |
Methyl 2-octynoate Chemical Properties
IUPAC: Methyl oct-2-ynoate
Methyl 2-octynoate (111-12-6) is also called for 2-Octynoic acid, methyl ester ; 4-02-00-01715 (Beilstein Handbook Reference) ; AI3-03938 ; BRN 1756887 ; EINECS 203-836-6 ; FEMA No. 2729 ; Folione ; Methyl 2-octinate ; Methyl 2-octynate ; Methyl hept-1-yne-1-carboxylate ; Methyl heptine carbonate ; Methyl pentylacetylenecarboxylate ; NSC 72098 ; Vert de violette, artificial and so on.
Molecular Formula: C9H14O2
Molecular Weight: 154.21
EINECS: 203-836-6
LogP: ACD/LogP: 3.56
XLogP: 3.30
ALOGPS: 3.08
ACD/LogD (pH 5.5): 3.56
ACD/LogD (pH 7.4): 3.56
ACD/BCF (pH 5.5): 299.29
ACD/BCF (pH 7.4): 299.29
ACD/KOC (pH 5.5): 2060.62
ACD/KOC (pH 7.4): 2060.62
H bond acceptors: 2
H bond donors: 0
Freely Rotating Bonds: 5
Heavy Atom Count: 11
Formal Charge: 0
Complexity: 171
Isotope Atom Count: 0
Defined Atom StereoCenter Count: 0
Undefined Atom StereoCenter Count: 0
Defined Bond StereoCenter Count: 0
Undefined Bond StereoCenter Count: 0
Covalently-Bonded Unit Count: 1
Index of Refraction: 1.443
Molar Refractivity: 43.52 cm3
Molar Volume: 163.8 cm3
Surface Tension: 33.1 dyne/cm
Density: 0.94 g/cm3
Flash Point: 88.9°C
Enthalpy of Vaporization: 45.49 kJ/mol
Boiling Point: 218.5°C at 760 mmHg
Vapour Pressure: 0.125 mmHg at 25°C
The molecular structure of Methyl 2-octynoate (111-12-6):
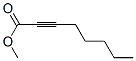
Methyl 2-octynoate Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MOD | FCTXAV Food and Cosmetics Toxicology. 17 (1979),375. | ||
| 2. | orl-rat LD50:1530 mg/kg | FCTXAV Food and Cosmetics Toxicology. 17 (1979),375. | ||
| 3. | skn-rbt LD50:3300 mg/kg | FCTXAV Food and Cosmetics Toxicology. 17 (1979),375. |
Methyl 2-octynoate Consensus Reports
Reported in EPA TSCA Inventory.
Methyl 2-octynoate Safety Profile
Moderately toxic by ingestion and skin contact. A moderate skin and eye irritant. A combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes: Xn ![]()
Risk Statements: 22-38
R22: Harmful if swallowed
R38: Irritating to the skin
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36: Wear suitable protective clothing
WGK Germany: 2
RTECS: RI2735000
Methyl 2-octynoate Specification
Treatment of Methyl 2-octynoate (111-12-6) in Wastewater,see as follows :
Total removal:8.15 percent
Total biodegradation:0.10 percent
Total sludge adsorption:3.20 percent
Total to air:4.85 percent
(using 10000 hr Bio P,A,S)
Related Products
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- Methyl (2R)-2-bromo-2-(2-chlorophenyl)acetate
- Methyl (2R,3S)-3-(4-methoxyphenyl)-2-oxiranecarboxylate
- Methyl (2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoate
- Methyl (2R,3S)-3-(tert-butoxycarbonylamino)-2-hydroxy-3-phenylpropionate
- Methyl (2S)-1-(1,2-dioxo-3,3-dimethypentyl)-2-pyrrolidinecarboxylate
- Methyl (2S)-2,3-epoxypropanoate
- 111128-12-2
- 111129-24-9
- 11113-50-1
- 11113-61-4
- 11113-63-6
- 11113-66-9
- 111-13-7
- 11113-75-0
- 11113-81-8
- 11113-82-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View