-
Name
Methyl butyrate
- EINECS 210-792-1
- CAS No. 623-42-7
- Article Data190
- CAS DataBase
- Density 0.891 g/cm3
- Solubility Slightly soluble in water.
- Melting Point -85--84°C
- Formula C5H10O2
- Boiling Point 104.2 °C at 760 mmHg
- Molecular Weight 102.133
- Flash Point 11.7 °C
- Transport Information UN 1237 3/PG 2
- Appearance colourless liquid
- Safety 16-26-36
- Risk Codes 20-36/37/38-11
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xn,
Xn,  Xi
Xi
- Synonyms Butyric acid, methyl ester (6CI,8CI);3-Methylpropanoic acid methyl ester;Methyl butanoate;Methyln-butyrate;NSC 9380;
- PSA 26.30000
- LogP 0.95950
Synthetic route
-
-
623-43-8, 4358-59-2, 18707-60-3, 130981-57-6
methyl crotonate

-
-
623-42-7
butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 80℃; under 37503.8 Torr; for 3h; Catalytic behavior; Reagent/catalyst; Temperature; Autoclave; | 100% |
| With 6C53H32O8(4-)*13Zr(4+)*18O(2-)*8Co(2+)*8H(1-); hydrogen In tetrahydrofuran at 20℃; under 30003 Torr; for 36h; Catalytic behavior; | 99% |
| With 6C53H32O8(4-)*13Zr(4+)*18O(2-)*8Co(2+)*8Cl(1-); hydrogen; sodium triethylborohydride In tetrahydrofuran at 23℃; under 30003 Torr; for 36h; Catalytic behavior; | 99% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; methylcopper; diisobutylaluminium hydride In tetrahydrofuran; diethyl ether; hexane at -50℃; for 0.5h; | 100% |
| With hydrogen; Rhodium chloride tri(triphenylphosphine-meta-trisulfonate) In water for 16h; Ambient temperature; | 95% |
| With hydrogen; Pd(II) complex containing tridentate hydrazonic ligands In methanol at 40℃; for 24h; Hydrogenation; | 35% |
| With hydrogen; montmorillonite-(bipyridine)x In benzene at 75℃; under 31028.9 Torr; for 45h; | 91 % Spectr. |
| With hydrogen In methanol at 80℃; under 37503.8 Torr; for 20h; Autoclave; |

| Conditions | Yield |
|---|---|
| With C61H98ClN3P2Ru In dichloromethane-d2 at 50℃; under 3040.2 Torr; for 4h; Reagent/catalyst; Time; | 100% |

| Conditions | Yield |
|---|---|
| With aluminum(III) sulphate octadecahydrate at 110℃; for 0.166667h; Sealed tube; Microwave irradiation; | 99.7% |
| With NiO/SiO2 at 360℃; Reagent/catalyst; | 97.98% |
| With 1-methyl-3-(4-sulfobutyl)-1H-imidazol-3-ium hydrogensulfate at 80℃; for 2h; | 96% |

| Conditions | Yield |
|---|---|
| With strontium hydroxide; dihexyl ether at 60℃; Reagent/catalyst; Concentration; Time; | 98% |
| With carbon-based sulfonated solid acid prepared at 150 °C at 80℃; for 8h; Catalytic behavior; Kinetics; Reagent/catalyst; | 97.2% |
| With Et3N-grafted carbon nanotubes at 60℃; for 8h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With triethylamine at 160℃; for 5h; Autoclave; Green chemistry; | 97% |
| With diiron nonacarbonyl at 180℃; for 1h; Sealed tube; |
-
-
67-56-1
methanol

-
-
187737-37-7
propene

-
-
201230-82-2
carbon monoxide

-
-
623-42-7
butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With bis(acetylacetonato)palladium(II); 1,1’-ferrocenediyl-bis(tert-butyl(pyridin-2-yl)phosphine); toluene-4-sulfonic acid at 23℃; for 0.166667h; Catalytic behavior; Temperature; Autoclave; Inert atmosphere; | 95% |
| With dodecacarbonyl-triangulo-triruthenium at 180℃; for 5h; Product distribution; solvents, water concentration, and added salts of group I-III, VI, or VIII dependence; |

-
-
24406-16-4
lithium di-n-butylcuprate

-
-
292638-85-8
acrylic acid methyl ester

-
-
623-42-7
butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In diethyl ether for 2h; -78 deg C to 20 deg C; | 87% |

| Conditions | Yield |
|---|---|
| With methanol; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In chloroform at 20℃; for 1h; | 85% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; sodium bromide at 20℃; for 2h; | 83% |
| With sodium carbonate; N,N'-diiodo-N,N'-1,2-ethanediylbis(p-toluenesulphonamide) at 20℃; for 36h; | 80% |
| Stage #1: methanol; butyraldehyde With tris(pentafluorophenyl)borate for 0.25h; Green chemistry; Stage #2: With tert.-butylhydroperoxide In decane for 24h; Green chemistry; | 72% |
-
-
67-56-1
methanol

-
-
187737-37-7
propene

-
-
201230-82-2
carbon monoxide

-
A
-
547-63-7
Methyl isobutyrate

-
B
-
623-42-7
butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; palladium diacetate; bis(phosphaadamantyl)diphosphine at 60℃; | A n/a B 81% |
| With hydrogenchloride; Pd(II) salt of poly(nornornaneketone)carboxylic acid; triphenylphosphine In xylene at 100℃; under 22501.8 Torr; Product distribution; | |
| With hydrogenchloride; bis-triphenylphosphine-palladium(II) chloride In xylene at 99.85℃; under 26252.1 - 30002.4 Torr; | |
| With hydrogenchloride; bis-triphenylphosphine-palladium(II) chloride In methanol at 99.85℃; under 26252.1 Torr; Rate constant; Product distribution; Mechanism; other Pd-catalst system; other solvent, temperature and pressure, also in the presence of var. Co, Fe, Cu additives as promoters; | |
| With C39H34O2P2; palladium(II) acetylacetonate; toluene-4-sulfonic acid In diethylene glycol dimethyl ether at 100℃; under 30003 Torr; for 4h; Catalytic behavior; Reagent/catalyst; Pressure; Temperature; Autoclave; |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In methanol at 20℃; for 0.25h; | 81% |
-
-
108-24-7
acetic anhydride

-
-
107-87-9
2-Pentanone

-
A
-
554-12-1
propanoic acid methyl ester

-
B
-
623-42-7
butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With oxygen; manganese(III) triacetate dihydrate at 90℃; under 3750.38 Torr; for 10h; Autoclave; | A 21% B 79% |


| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; water In methanol at 20℃; for 0.5h; | 78% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; polystyrene-bound phenylseleninic acid for 8h; Heating; | 75% |
-
-
591-78-6
n-hexan-2-one

-
-
108-24-7
acetic anhydride

-
A
-
623-42-7
butanoic acid methyl ester

-
B
-
624-24-8
methyl valerate

| Conditions | Yield |
|---|---|
| With oxygen; manganese(III) triacetate dihydrate at 90℃; under 3750.38 Torr; for 10h; Autoclave; | A 20% B 72% |

-
-
67-56-1
methanol

-
-
106-31-0
butanoic acid anhydride

-
-
27126-76-7
[hydroxy(tosyloxy)iodo]benzene

-
A
-
591-50-4
iodobenzene

-
B
-
623-42-7
butanoic acid methyl ester

-
C
-
118921-64-5
methyl-2-(tosyloxy)butyrate

| Conditions | Yield |
|---|---|
| Stage #1: butanoic acid anhydride; [hydroxy(tosyloxy)iodo]benzene at 100℃; for 0.333333h; Stage #2: methanol With toluene-4-sulfonic acid for 4h; Heating; | A n/a B n/a C 61% |

-
-
138723-86-1
2-methoxy-2-n-propyl-5,5-pentamethylene-1,3,4-Δ3-oxadiazoline

-
A
-
623-42-7
butanoic acid methyl ester

-
B
-
108-94-1
cyclohexanone

-
C
-
138724-04-6
1-(1-Methoxy-butoxy)-cyclohexene

-
D
-
110-83-8
cyclohexene

| Conditions | Yield |
|---|---|
| for 1h; Heating; | A 39% B 13% C 50% D 32% |


| Conditions | Yield |
|---|---|
| With CuO on mesoporous silica at 360℃; Temperature; | A 45.99% B 28.72% |


| Conditions | Yield |
|---|---|
| With Au#Co; oxygen; potassium carbonate at 130℃; under 4500.45 Torr; for 7h; Autoclave; | 45% |
| With oxygen; potassium carbonate at 130℃; under 4560.31 Torr; for 7h; | |
| With oxygen; potassium carbonate at 45℃; for 12h; Green chemistry; | 41 %Chromat. |
| With Au/CeO2; oxygen; caesium carbonate at 25℃; under 760.051 Torr; for 24h; Reagent/catalyst; | |
| With bromine at 40℃; Temperature; | 93 %Chromat. |
-
-
67-56-1
methanol

-
-
1942-45-6
4-Octyne

-
A
-
5455-24-3
octane-4,5-dione

-
B
-
4643-25-8
hept-2-en-4-one

-
C
-
623-42-7
butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methyltrioxorhenium(VII) for 48h; | A 41% B 17% C 15% |

-
-
1942-45-6
4-Octyne

-
A
-
5455-24-3
octane-4,5-dione

-
B
-
4643-25-8
hept-2-en-4-one

-
C
-
623-42-7
butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; methyltrioxorhenium(VII) In methanol for 48h; | A 41% B 17% C 15% |

-
-
67-56-1
methanol

-
-
142-96-1
dibutyl ether

-
A
-
4461-87-4
1,1-dimethoxybutane

-
B
-
623-42-7
butanoic acid methyl ester

-
C
-
113443-65-5
1-n-butoxy-1-methoxybutane

| Conditions | Yield |
|---|---|
| With tris (2,4-dibromophenyl)amine; sodium methylate; lithium perchlorate at 40℃; electrochem. oxidation; | A 7.5% B 7.5% C 26% |


-
-
79060-88-1
sodium tetrakis[(3,5-di-trifluoromethyl)phenyl]borate

-
-
292638-85-8
acrylic acid methyl ester

-
A
-
623-42-7
butanoic acid methyl ester

-
B
-
3724-55-8
methyl but-3-enoate

-
D
-
7440-05-3
palladium

| Conditions | Yield |
|---|---|
| In dichloromethane for 5h; Schlenk technique; Inert atmosphere; Darkness; | A n/a B n/a C 20% D n/a |


-
-
79060-88-1
sodium tetrakis[(3,5-di-trifluoromethyl)phenyl]borate

-
-
292638-85-8
acrylic acid methyl ester

-
A
-
623-42-7
butanoic acid methyl ester

-
B
-
3724-55-8
methyl but-3-enoate


-
D
-
7440-05-3
palladium

| Conditions | Yield |
|---|---|
| In dichloromethane for 5h; Schlenk technique; Inert atmosphere; Darkness; | A n/a B n/a C 19% D n/a |


| Conditions | Yield |
|---|---|
| With tris (2,4-dibromophenyl)amine; sodium methylate; lithium perchlorate at 40℃; electrochem. oxidation; | 12.2% |
-
-
67-56-1
methanol

-
-
24072-44-4
Cyclohexyl-n-butyl ether

-
A
-
933-40-4
cycloxexanone dimethyl ketal

-
B
-
4461-87-4
1,1-dimethoxybutane

-
C
-
623-42-7
butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With tris (2,4-dibromophenyl)amine; sodium methylate; lithium perchlorate at 40℃; electrochem. oxidation; | A 3.6% B 11% C 2.3% |


| Conditions | Yield |
|---|---|
| With sodium chloride at 49.84 - 89.84℃; for 6h; | 10.9% |
| In water at 25℃; Kinetics; pH 7; reaction in presence of enzyme extract from Brevibacterium sp. R312 or Brevibacterium linens 62; | |
| With C7H10N2O2*Zn(2+)*2C7H9N2O2(1-)*H2O at 75℃; for 24h; Catalytic behavior; | 37 %Spectr. |
-
-
2026-48-4
(S)-valinol

-
-
623-42-7
butanoic acid methyl ester

-
-
906672-84-2
(S)-2-butyl-4-isopropyloxazoline

| Conditions | Yield |
|---|---|
| Zn4(OCOCF3)6O In chlorobenzene for 12h; Product distribution / selectivity; Heating / reflux; | 100% |
| Zn4(OAc)6O In chlorobenzene for 12h; Product distribution / selectivity; Heating / reflux; | 84% |
-
-
623-42-7
butanoic acid methyl ester

-
-
140-29-4
phenylacetonitrile

-
-
105909-68-0
3-oxo-2-phenyl-hexanenitrile

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0 - 85℃; for 21h; | 99% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate at 85℃; under 52.5053 Torr; for 3.5h; Large scale; | 98.4% |
-
-
623-42-7
butanoic acid methyl ester

-
-
112-27-6
2,2'-[1,2-ethanediylbis(oxy)]bisethanol

-
-
26962-26-5
triethylene glycol di-n-butyrate

| Conditions | Yield |
|---|---|
| With potassium carbonate at 100 - 110℃; | 98% |
-
-
623-42-7
butanoic acid methyl ester

-
-
29601-98-7
N-benzylhydroxylamine hydrochloride

-
-
1206188-41-1
(E)-N-(1-methoxy-1-oxobutan-2-ylidene)(phenyl)methanamine N-oxide

| Conditions | Yield |
|---|---|
| With sodium acetate In methanol; water at 20℃; for 16h; | 95% |

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide; triphenylphosphine; copper(I) bromide In diethyl ether for 4h; Heating; | 93% |


| Conditions | Yield |
|---|---|
| at 110℃; for 6h; | 92% |
-
-
623-42-7
butanoic acid methyl ester

-
-
105224-90-6
dimethylaminobis(trifluoromethyl)borane

-
-
148298-54-8
1-methoxycarbonyl-n-propyl-bis(trifluoromethyl)borane-dimethylamine

| Conditions | Yield |
|---|---|
| In pentane dropwise addn. of (CF3)2BNMe2 to a stirred soln. of the carbonyl compound in dry pentane at 4°C; warmed to room temp. with stirring (1 h);; removal of solvent and volatile by-products in vac. at room temp., purifn. by sublimation in vac.; elem. anal.;; | 92% |
-
-
623-42-7
butanoic acid methyl ester

-
-
925-90-6
ethylmagnesium bromide

-
-
40122-38-1
1-propyl-1-cyclopropanol

| Conditions | Yield |
|---|---|
| titanium(IV) isopropylate In diethyl ether at 18 - 20℃; | 91% |
| With titanium(IV) isopropylate In diethyl ether at 18 - 20℃; | 91% |
| With titanium(IV) isopropylate In diethyl ether at -78 - 0℃; for 0.5h; | 90% |
-
-
623-42-7
butanoic acid methyl ester

-
-
18854-66-5
tris(allyl)aluminum

-
-
52939-61-4
4-hydroxy-4-n-propyl-1,6-heptadiene

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 0.5h; | 91% |
-
-
623-42-7
butanoic acid methyl ester

-
-
96797-15-8
N-trityl-4(5)-iodoimidazole

-
-
1258289-19-8
1,1-bis(1-trityl-1H-imidazol-4-yl)butan-1-ol

| Conditions | Yield |
|---|---|
| Stage #1: N-trityl-4(5)-iodoimidazole With ethylmagnesium bromide In diethyl ether; dichloromethane at 20℃; for 2h; Double Grignard reaction; Inert atmosphere; Stage #2: butanoic acid methyl ester In diethyl ether; dichloromethane at 20℃; for 48h; Double Grignard reaction; Inert atmosphere; Stage #3: With water; ammonium chloride In diethyl ether; dichloromethane | 90% |

| Conditions | Yield |
|---|---|
| With zeolite at 115 - 120℃; for 12h; Dean-Stark; | 90% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate for 5h; Heating; | 89% |
| With hydrazine hydrate | |
| With hydrazine hydrate at 90℃; for 1h; Microwave irradiation; | |
| With hydrazine In ethanol Reflux; | |
| With hydrazine In water at 70℃; for 0.75h; |
-
-
623-42-7
butanoic acid methyl ester

-
-
100-58-3
phenylmagnesium bromide

-
-
5331-17-9
1,1-diphenylbutan-1-ol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20 - 50℃; for 16h; Inert atmosphere; Schlenk technique; | 89% |
| Stage #1: butanoic acid methyl ester; phenylmagnesium bromide In tetrahydrofuran at 60℃; for 3h; Inert atmosphere; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 20℃; Inert atmosphere; | |
| In tetrahydrofuran; 2-methyltetrahydrofuran at -10 - 25℃; for 6.25h; Schlenk technique; Inert atmosphere; |
-
-
623-42-7
butanoic acid methyl ester

-
-
756-79-6
dimethyl methane phosphonate

-
-
65921-74-6
dimethyl (2-oxopentyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl methane phosphonate With n-butyllithium In tetrahydrofuran; hexane at -90℃; for 0.5h; Inert atmosphere; Stage #2: butanoic acid methyl ester In tetrahydrofuran; hexane at -90 - 20℃; Inert atmosphere; | 89% |
-
-
623-42-7
butanoic acid methyl ester

-
-
2393-23-9
4-methoxy-benzylamine

-
-
430447-31-7
N-(4-methoxybenzyl)butyramide

| Conditions | Yield |
|---|---|
| With calcium iodide In toluene at 110℃; for 4h; Green chemistry; chemoselective reaction; | 89% |

| Conditions | Yield |
|---|---|
| With calcium iodide In toluene at 110℃; for 4h; Reagent/catalyst; Green chemistry; chemoselective reaction; | 89% |
| With zirconocene dichloride In toluene at 110℃; for 4h; | 70% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 103℃; Inert atmosphere; Schlenk technique; | 89% |

| Conditions | Yield |
|---|---|
| With Sn[N(SO2C8F17)2]4 In toluene at 80℃; for 15h; | 88% |
| With Sn[N(SO2C8F17)2]4; octadecafluorodecahydronaphthalene (cis+trans) In toluene at 80℃; for 15h; | 89 % Chromat. |
-
-
623-42-7
butanoic acid methyl ester

-
-
1207973-75-8
lithium tert-butyl acetate

-
-
61540-30-5
3-oxohexanoic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| 88% |
-
-
623-42-7
butanoic acid methyl ester

-
-
97968-16-6
1-(4'-methoxyphenyl)-pentan-2-one

| Conditions | Yield |
|---|---|
| With lithium tert-butoxide In tetrahydrofuran at 23℃; for 3h; Glovebox; | 88% |
-
-
623-42-7
butanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With lithium tert-butoxide In tetrahydrofuran at 23℃; for 3h; Glovebox; | 88% |
Methyl butyrate Consensus Reports
Methyl butyrate Standards and Recommendations
Methyl butyrate Specification
The Methyl-n-butyrate with CAS registry number of 623-42-7 is also called Butanoicacid, methyl ester. The IUPAC name is methyl butanoate. Its EINECS registry number is 210-792-1. In addition, the molecular formula is C5H10O2 and the molecular weight is 102.1317. It is a kind of colourless liquid and belongs to the classes of Organics; Analytical Chemistry; Fatty Acid Methyl Esters (GC Standard); Standard Materials for GC; C2 to C5Saturated fatty acids and derivatives; Carbonyl Compounds; Methyl Esters; Alphabetical Listings; Certified Natural ProductsFlavors and Fragrances; Flavors and Fragrances.
Physical properties about this chemical are: (1)ACD/LogP: 1.24; (2)ACD/LogD (pH 5.5): 1.24; (3)ACD/LogD (pH 7.4): 1.24; (4)ACD/BCF (pH 5.5): 5.15; (5)ACD/BCF (pH 7.4): 5.15; (6)ACD/KOC (pH 5.5): 112.56; (7)ACD/KOC (pH 7.4): 112.56; (8)#H bond acceptors: 2 ; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.387; (12)Molar Refractivity: 26.98 cm3; (13)Molar Volume: 114.5 cm3; (14)Polarizability: 10.69 ×10-24cm3; (15)Surface Tension: 24.8 dyne/cm; (16)Density: 0.891 g/cm3; (17)Flash Point: 11.7 °C; (18)Enthalpy of Vaporization: 34.33 kJ/mol; (19)Boiling Point: 104.2 °C at 760 mmHg; (20)Vapour Pressure: 31.1 mmHg at 25°C.
Preparation of Methyl-n-butyrate: it can be prepared by methanol and butyric acid through esterification reaction in presence of catalyst sulfuric acid. The product often contain free butyric acid and methanol by hydrolysis. In order to refined it, you can use potassium bicarbonate solution to wash it.
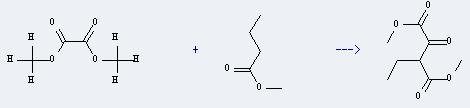
Uses of Methyl-n-butyrate: except for resin and paint solvents, it also can be used as raw materials of man-made rum and fruit flavors. In addition, it can react with oxalic acid dimethyl ester to get methyl 3-ethyl-2-oxobutane-1,4-dioate. The yield is about 70%.
When you are using this chemical, please be cautious about it as the following:
It is highly Flammable and harmful by inhalation. In addition, it is irritating to eyes, respiratory system and skin. During using it, you should wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. And keep it away from sources of ignition-No smoking.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OC)CCC
(2)InChI: InChI=1/C5H10O2/c1-3-4-5(6)7-2/h3-4H2,1-2H3
(3)InChIKey: UUIQMZJEGPQKFD-UHFFFAOYAW
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LC50 | inhalation | 20gm/m3 (20000mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 51(5), Pg. 61, 1986. | |
| mouse | LC50 | inhalation | 18gm/m3/2H (18000mg/m3) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 81, 1982. | |
| rabbit | LD50 | oral | 3380mg/kg (3380mg/kg) | Industrial Medicine and Surgery. Vol. 41, Pg. 31, 1972. | |
| rabbit | LD50 | skin | 3560mg/kg (3560mg/kg) | Toxicology and Applied Pharmacology. Vol. 42, Pg. 417, 1977. | |
| rat | LD50 | oral | > 5gm/kg (5000mg/kg) | Food and Chemical Toxicology. Vol. 20, Pg. 741, 1982. |
Related Products
- Methyl 1-Benzyl-5-oxopyrrolidine-3-carboxylate
- Methyl (((methoxymethylphosphinothioyl)thio)acetyl)methylcarbamate
- Methyl (+)-(3R)-7-[4-(4-fluorophenyl)-6-isopropyl-2-(N-methyl-N-methanesulfonylamino)pyrimidin-5-yl]-3-hydroxy-5-oxo-(6E)-heptenoate
- Methyl (2-amino-5-methyl-1,3-thiazol-4-yl)acetate
- Methyl (2-chloromethyl)oxazole-4-carboxylate
- Methyl (2E)-3-(4-methylphenyl)propenoate
- Methyl (2E)-3-cyclohexylprop-2-enoate
- Methyl (2R)-2-[(tert-butoxycarbonyl)amino]-3-iodopropanoate
- Methyl (2R)-2-[4-(2,4-dichlorophenoxy)phenoxy]propanoate
- Methyl (2R)-2-amino-2-cyclohexylethanoate hydrochloride
- 623-43-8
- 62345-76-0
- 623-46-1
- 623-47-2
- 62348-13-4
- 623-48-3
- 623-49-4
- 623-50-7
- 62351-47-7
- 623-51-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View