-
Name
Diethanolmethylamine
- EINECS 203-312-7
- CAS No. 105-59-9
- Article Data33
- CAS DataBase
- Density 1.051 g/cm3
- Solubility miscible with water
- Melting Point -21 °C
- Formula C5H13NO2
- Boiling Point 247 °C at 760 mmHg
- Molecular Weight 119.164
- Flash Point 126.7 °C
- Transport Information UN 2735
- Appearance colourless viscous liquid
- Safety 24
- Risk Codes 36
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Ethanol,2,2'-(methylimino)di- (6CI,8CI);2,2'-(Methylimino)bis[ethanol];2,2'-(Methylimino)diethanol;2-[(2-Hydroxyethyl)(methyl)amino]ethanol;AminoAlcohol MDA;Diethanolmethylamine;Gas Spec CS 2000;Jefftreat MS 100;MDEA;MDEA (diol);Methylbis(2-hydroxyethyl)amine;Methyldiethanolamine;Methyliminodiethanol;N,N-Bis(2-hydroxyethyl)methylamine;N,N-Di(2-hydroxyethyl)-N-methylamine;N,N-Di(2-hydroxyethyl)methylamine;N,N-Di-(2-hydroxyethyl)-methanamine;N-(2-Hydroxyethyl)-N-methylethanolamine;N-Methyl-N,N-Bis(2-hydroxyethyl)amine;N-Methyl-N,N-diethanolamine;N-Methylbis(2-hydroxyethyl)amine;N-Methyliminodiethanol;NSC 11690;NSC 49131;NSC 51500;
- PSA 43.70000
- LogP -1.09720
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen In methanol at 125℃; under 10501.1 Torr; for 4h; Autoclave; | 92% |
| und Hydrierung des Reaktionspodukts an Raney-Nickel bei 85grad/175 at; |
-
-
75-21-8
oxirane

-
-
74-89-5
methylamine

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
105-59-9
N-Methyldiethanolamine

-
C
-
68998-54-9
2,2'-((1-hydroxypropan-2-yl)azanediyl)bis(ethan-1-ol)

| Conditions | Yield |
|---|---|
| water at 62.84 - 71.84℃; under 7500.75 Torr; for 0.2h; Heating / reflux; | A 16.7% B 83.3% C n/a |
| at 66.84 - 76.84℃; under 9000.9 Torr; for 0.116667h; Heating / reflux; | A 63.5% B 36.4% C 0.02% |
| water at 61.54 - 63.34℃; under 7500.75 Torr; for 0.2h; Heating / reflux; | |
| at 66.34℃; under 9000.9 Torr; for 0.116667h; Heating / reflux; |


| Conditions | Yield |
|---|---|
| With methanol; nickel at 95 - 100℃; under 514855 Torr; |


| Conditions | Yield |
|---|---|
| With hydrogen; palladium/alumina at 290℃; for 5h; Product distribution; various temp., time, catalysts; | |
| With hydrogen; Pt/Al2O3 at 320℃; for 5h; |
-
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
-
111-44-4
3-oxa-1,5-dichloropentane

-
A
-
105-59-9
N-Methyldiethanolamine

-
B
-
115188-50-6
3,9-dimethyl-6-oxa-3,9-diaza-undecane-1,11-diol

| Conditions | Yield |
|---|---|
| With sodium carbonate In toluene for 48h; Heating; | A 14% B 37% |


| Conditions | Yield |
|---|---|
| With water at 120℃; |
-
-
55-86-7
mechlorethamine hydrochloride

-
A
-
105-59-9
N-Methyldiethanolamine

-
B
-
111068-26-9
bis-(2-hydroxy-ethyl)-{2-[(2-hydroxy-ethyl)-methyl-amino]-ethyl}-methyl-ammonium; chloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium carbonate In water-d2 for 24h; Title compound not separated from byproducts; |
-
-
65796-77-2
N-methyldiethanolamine phenylboronic ester

-
A
-
105-59-9
N-Methyldiethanolamine

-
B
-
98-80-6
phenylboronic acid

| Conditions | Yield |
|---|---|
| With water In 1,4-dioxane; water Kinetics; byproducts: (HOCH2CH2)2NMe; hydrolysis in water/dioxane mixts. of different concns.; |
-
-
20073-50-1
2-(oxazolidin-3-yl)ethanol

-
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| With formic acid; water | |
| With formic acid; water |

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| at 100 - 110℃; |

| Conditions | Yield |
|---|---|
| at 160 - 165℃; |

| Conditions | Yield |
|---|---|
| at 100℃; |
-
-
50-00-0
formaldehyd

-
-
64-18-6
formic acid

-
-
111-42-2
2,2'-iminobis[ethanol]

-
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| With water zuletzt auf dem Dampfbad; |

-
-
70-18-8
GLUTATHIONE

-
-
51-75-2
nitrogen mustard

-
A
-
105-59-9
N-Methyldiethanolamine


-
C
-
124605-74-9
(S)-2-Amino-4-[(R)-2-[2-({2-[(R)-2-((S)-4-amino-4-carboxy-butyrylamino)-2-(carboxymethyl-carbamoyl)-ethylsulfanyl]-ethyl}-methyl-amino)-ethylsulfanyl]-1-(carboxymethyl-carbamoyl)-ethylcarbamoyl]-butyric acid

| Conditions | Yield |
|---|---|
| In water-d2 at 30℃; for 3.06667h; Mechanism; 0.06 M phosphate, pH 7.0, NMR characterization; |

-
-
92336-62-4
Ethoxy-bis(2-hydroxyethyl)methylammonium

-
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| (i) AgCl, (ii) H2, Pd-C; Multistep reaction; |
-
-
67-56-1
methanol

-
-
50-00-0
formaldehyd

-
-
107-16-4
glycolonitrile

-
A
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
B
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| at 90 - 110℃; under 514855 Torr; Hydrogenation; |

-
-
67-56-1
methanol

-
-
50-00-0
formaldehyd

-
-
107-16-4
glycolonitrile

-
A
-
108-01-0
2-(N,N-dimethylamino)ethanol

-
B
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| at 90 - 110℃; under 514855 Torr; Hydrogenation; |

-
-
7732-18-5
water

-
-
51-75-2
nitrogen mustard

-
A
-
105-59-9
N-Methyldiethanolamine

-
B
-
51822-57-2
2‐[(2-chloroethyl)(methyl)amino]ethanol

| Conditions | Yield |
|---|---|
| wandelt sich bei Raumtemperatur nach mehreren Tagen; |

-
-
75-58-1
tertamethylammonium iodide

-
-
111-42-2
2,2'-iminobis[ethanol]

-
A
-
105-59-9
N-Methyldiethanolamine

-
B
-
75-50-3
trimethylamine

| Conditions | Yield |
|---|---|
| at 154℃; Rate constant; |

-
-
75-21-8
oxirane

-
-
74-89-5
methylamine

-
A
-
109-83-1
(2-hydroxyethyl)(methyl)amine

-
B
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| unter starker Kuehlung; |

-
-
105-59-9
N-Methyldiethanolamine

-
-
652148-91-9
(5-chloropyridin-2-yl)boronic acid

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 18h; | 100% |
-
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 18h; | 100% |
-
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| In benzene under Ar atm. mixt. MeN(CH2CH2OH)2, Ge(OMe)4, and benzene was refluxed for 2 h; volatiles were removedin vacuo; elem. anal.; | 100% |
-
-
14103-74-3
bis(dimethylamino)dimethylgermane

-
-
105-59-9
N-Methyldiethanolamine

-
-
722458-51-7
2,2,6-trimethyl-1,3-dioxa-6-aza-2-germacyclooctane

| Conditions | Yield |
|---|---|
| In toluene byproducts: HNMe2; under Ar, stirring at 70 °C for 10 h; volatiles were removed in vac., elem. anal.; | 100% |
-
-
105-59-9
N-Methyldiethanolamine

-
-
1437769-74-8
(2-{[(trifluoromethyl)sulfonyl]oxy}phenyl)boronic acid

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With sodium iodide In acetonitrile for 96h; Reflux; | 100% |
-
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 3h; Inert atmosphere; Molecular sieve; | 100% |
-
-
105-59-9
N-Methyldiethanolamine

| Conditions | Yield |
|---|---|
| In benzene at 100℃; for 4h; Molecular sieve; | 100% |

| Conditions | Yield |
|---|---|
| In acetone at 35 - 45℃; | 100% |

| Conditions | Yield |
|---|---|
| In acetone at 40℃; | 100% |

| Conditions | Yield |
|---|---|
| In toluene at 20℃; for 3h; Inert atmosphere; Schlenk technique; Glovebox; | 100% |
-
-
105-59-9
N-Methyldiethanolamine

-
-
74-88-4
methyl iodide

-
-
863031-19-0
di(methoxyethyl)dimethylammonium iodide

| Conditions | Yield |
|---|---|
| Stage #1: N-Methyldiethanolamine With sodium hydride In tetrahydrofuran at 25 - 40℃; for 0.5h; Stage #2: methyl iodide at 40℃; for 7h; | 99.2% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 99% |
| at 20℃; for 12h; Cooling with ice; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: N-Methyldiethanolamine With sulfuric acid at 60 - 170℃; for 7.5h; Stage #2: at 60 - 70℃; for 0.5h; Temperature; Reagent/catalyst; Time; | 98.4% |
| With hydrogenchloride at 160℃; im Rohr; Uebersaettigen mit Alkalilauge und Destillieren mit Wasserdampf; | |
| With sulfuric acid at 160℃; Uebersaettigen mit Alkalilauge und Destillieren mit Wasserdampf; | |
| With aluminum oxide; silica gel at 400℃; | |
| With rare-earth based solid catalyst at 200℃; Temperature; |

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane at 0 - 20℃; for 2h; Inert atmosphere; Schlenk technique; | 98% |
| With thionyl chloride; benzene zuletzt bei Siedetemperatur; | |
| With thionyl chloride; chloroform zuletzt bei Siedetemperatur; |
-
-
105-59-9
N-Methyldiethanolamine

-
-
440680-34-2
(6-bromopyridin-2-yl)boronic acid

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; for 18h; | 98% |
-
-
1020-31-1
3,5-Di-tert-butylcatechol

-
-
105-59-9
N-Methyldiethanolamine

-
-
128426-02-8
tetrapropyloxygermane

-
-
134576-38-8
((CH3)3C)2C6H2O2Ge(OCH2CH2)2NCH3

| Conditions | Yield |
|---|---|
| In benzene byproducts: CH3CH2CH2OH; refluxing a mixt. of tetrapropoxygermane, corresponding α-diol and diethanolamine in benzene (2-4 h), cooling, crystn.; filtration, washing (benzene, ether), drying (vac.); evapn. of the filtrate (vac.) down to half bulk, isolation of a further amt. of the product; elem. anal.; | 98% |

-
-
105-59-9
N-Methyldiethanolamine

-
-
3405-89-8
2-(4-chloro-phenylsulfonyl)acetic acid

-
-
1448165-12-5
N,N-bis(2-hydroxyethyl)-N-methylammonium 2-(4-chlorophenylsulfonyl)acetate

| Conditions | Yield |
|---|---|
| 98% | |
| at 25℃; | 95% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 40℃; Inert atmosphere; | 98% |
-
-
105-59-9
N-Methyldiethanolamine

-
-
66566-80-1
chloro(propylthio)acetylene

-
-
97427-21-9
3-(2-Hydroxy-ethyl)-3-methyl-2-[1-propylsulfanyl-meth-(E)-ylidene]-oxazolidin-3-ium; chloride

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 3h; | 97% |
-
-
105-59-9
N-Methyldiethanolamine

-
-
13331-27-6
m-nitrobenzene boronic acid

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 1h; | 97% |

| Conditions | Yield |
|---|---|
| In toluene; acetonitrile at 100℃; for 24h; | 97% |
-
-
105-59-9
N-Methyldiethanolamine

-
-
58567-81-0
N-methyl-N-2-hydroxyethyl-2-aminoethanol-N-oxide

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide | 96% |
| With dihydrogen peroxide |

| Conditions | Yield |
|---|---|
| With thionyl chloride In chloroform at 20℃; for 2h; | 96% |
| With thionyl chloride In dichloromethane at 0 - 20℃; for 24h; | 92% |
| With thionyl chloride In dichloromethane at 20℃; for 48h; Cooling with ice; | 85% |
-
-
105-59-9
N-Methyldiethanolamine

-
-
1107-00-2
4,4'-(1,1,1,3,3,3-hexafluoroisopropylidene)diphthalic anhydride

-
-
101-68-8
di(4-isocyanatophenyl)methane

- polymer, product of diol-diisocyanate condensation followed by polycondensation with dianhydride and heating; monomer(s): 4,4\-methylenebis(phenyl isocyanate); N-methyldiethanolamine; 4,4\-(hexafluoroisopropylidene)bis(phthalic anhydride)
-
polymer, product of diol-diisocyanate condensation followed by polycondensation with dianhydride and heating; monomer(s): 4,4\-methylenebis(phenyl isocyanate); N-methyldiethanolamine; 4,4\-(hexafluoroisopropylidene)bis(phthalic anhydride)

| Conditions | Yield |
|---|---|
| Stage #1: N-Methyldiethanolamine; di(4-isocyanatophenyl)methane In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 4,4'-(1,1,1,3,3,3-hexafluoroisopropylidene)diphthalic anhydride In N,N-dimethyl-formamide at 60 - 80℃; Stage #3: for 15h; Heating; vacuum; | 96% |


| Conditions | Yield |
|---|---|
| In toluene byproducts: H2O; under N2, 2 equiv. of N-compd., reflux in dry toluene with removing of water (with a mol. sieve) for ca. 4 h; volatiles were removed in vac., solid was recrystd. from hot toluene on slow cooling to -22 °C, elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| In toluene at 100℃; for 1h; | 96% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; | 95% |
| With potassium hydroxide | |
| In N,N-dimethyl-formamide at 20℃; for 24h; |
-
-
112-16-3
n-dodecanoyl chloride

-
-
105-59-9
N-Methyldiethanolamine

-
-
79898-78-5
bis<2-(dodecyloxycarbonyl)ethyl>methylamine hydrochloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide | 95% |
N-Methyldiethanolamine Consensus Reports
N-Methyldiethanolamine Specification
The CAS registry number of N-Methyldiethanolamine is 105-59-9. Its EINECS registry number is 203-312-7. The IUPAC name is 2-[2-hydroxyethyl(methyl)amino]ethanol. In addition, the molecular formula is C5H13NO2. What's more, it is a colourless viscous liquid and soluble in water. It is mainly used as emulsifier and acid gas absorbent, pH control agents, polyurethane foam catalyst. Besides, it is also used as an intermediate for hydrochloric acid chlormethine which is one of anticancer drugs.
Physical properties about this chemical are: (1)ACD/LogP: -0.72; (2)ACD/LogD (pH 5.5): -3.49; (3)ACD/LogD (pH 7.4): -1.89; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 3; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 6; (11)Polar Surface Area: 21.7 Å2; (12)Index of Refraction: 1.476; (13)Molar Refractivity: 32 cm3; (14)Molar Volume: 113.3 cm3; (15)Polarizability: 12.68 ×10-24cm3; (16)Surface Tension: 43 dyne/cm; (17)Density: 1.051 g/cm3; (18)Flash Point: 126.7 °C; (19)Enthalpy of Vaporization: 56.25 kJ/mol; (20)Boiling Point: 247 °C at 760 mmHg; (21)Vapour Pressure: 0.00431 mmHg at 25°C.
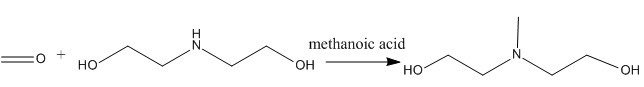
Preparation of N-Methyldiethanolamine: it can be prepared by methanal and diethanolamine. Add methanoic acid into the reactor and heat it to boiling at first. Then add methanal and diethanolamine with stirring in 1 hour. The reaction temperature should be maintained at 90-98 °C. In addition, the reaction should reflux for 4 hours. At last, you should collect 120-130 °C(0.53 kPa) fractions through vacuum distillation.
Uses of N-Methyldiethanolamine: it can be used to get 4-methyl-morpholin-2-one. This reaction will need reagents RuH2(PPh3)4 and acetone, and solvent toluene. The reaction time is 3 hours at reaction temperature of 180 °C. The yield is about 95%.

When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes. So during using it, you should avoid contact with skin.
You can still convert the following datas into molecular structure:
(1)SMILES: OCCN(C)CCO
(2)InChI: InChI=1/C5H13NO2/c1-6(2-4-7)3-5-8/h7-8H,2-5H2,1H3
(3)InChIKey: CRVGTESFCCXCTH-UHFFFAOYAC
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 500mg/kg (500mg/kg) | National Technical Information Service. Vol. AD277-689, | |
| rabbit | LD50 | skin | 5990uL/kg (5.99mL/kg) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 10, Pg. 61, 1954. | |
| rat | LC | inhalation | > 6500ug/m3/6H (6.5mg/m3) | Journal of Toxicology, Cutaneous and Ocular Toxicology. Vol. 17, Pg. 179, 1990. | |
| rat | LD50 | oral | 1945mg/kg (1945mg/kg) | SENSE ORGANS AND SPECIAL SENSES: CHROMODACYRORREA: EYE BEHAVIORAL: ATAXIA GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Veterinary and Human Toxicology. Vol. 38, Pg. 422, 1996. |
Related Products
- N-Methyldiethanolamine
- 1055995-89-5
- 105601-04-5
- 10560-13-1
- 1056016-06-8
- 1056016-18-2
- 1056016-74-0
- 105-60-2
- 10560-28-8
- 10561-02-1
- 105612-50-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View