-
Name
m-Cresol
- EINECS 203-577-9
- CAS No. 108-39-4
- Article Data522
- CAS DataBase
- Density 1.038 g/cm3
- Solubility 20 g/L (20 °C) in water
- Melting Point 8-10 °C(lit.)
- Formula C7H8O
- Boiling Point 202.279 °C at 760 mmHg
- Molecular Weight 108.14
- Flash Point 86.111 °C
- Transport Information UN 2076 6.1/PG 2
- Appearance colourless to light yellow liquid
- Safety 36/37/39-45-36/37
- Risk Codes 24/25-34-39/23/24/25-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms 3-Hydroxytoluene;3-Methylphenol;Caswell No. 261A;FEMA No. 3530;Franklin Cresolis;PHENOL;m-Cresol;
- PSA 20.23000
- LogP 1.70060
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonium formate; palladium on activated charcoal In methanol for 2h; Ambient temperature; | 100% |
| With 20 % Pd(OH)2/C; hydrogen In methanol at 20℃; under 760.051 Torr; for 3h; | 70% |
-
-
22719-82-0
ethyl (m-tolyl)carbonate

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With sodium hydrogen telluride In ethanol for 0.5h; Quantum yield; Heating; buffer: deoxygen. acetic acid; | 100% |
-
-
17902-31-7
(3-methylphenoxy)trimethylsilane

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With water; potassium carbonate In ethanol at 75℃; for 10h; | 100% |
| With Nanoporous Na+-Montmorillonite Perchloric Acid In ethanol at 20℃; for 0.05h; | 91% |
-
-
18406-01-4
triethyl(3-methylphenoxy)silane

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With water; potassium carbonate In ethanol at 75℃; for 10h; | 99% |
-
-
62790-75-4
1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-3-methylbenzene

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With water; potassium carbonate In ethanol at 75℃; for 12h; | 98% |
| With sodium cyanide In ethanol; water at 80℃; for 17h; chemoselective reaction; | 83.7% |
| With iodine In methanol for 22h; Ambient temperature; | 33% |
-
-
133921-27-4
4-iodo-3-methyl-phenol

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With potassium carbonate; isopropyl alcohol; palladium diacetate; triphenylphosphine at 90℃; for 14h; | 98% |
-
-
17933-03-8
m-tolylboronic acid

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide at 20℃; for 0.166667h; Green chemistry; | 98% |
| With sodium perborate tetrahydrate In neat (no solvent) at 25℃; for 0.166667h; Green chemistry; | 98% |
| With solid poly(N-vinylpyrrolidone)-hydrogen peroxide complex (PVD-H2O2 complex) In dichloromethane at 20℃; regioselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With ammonium bicarbonate In water at 20℃; for 2h; Schlenk technique; | 97% |
-
-
144265-47-4
tetrahydro-2-(3-methylphenoxy)-2H-pyran

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| Stage #1: tetrahydro-2-(3-methylphenoxy)-2H-pyran With aluminium(III) triflate In methanol at 20 - 25℃; for 2h; Inert atmosphere; Stage #2: With water; sodium hydrogencarbonate In methanol; dichloromethane at 20 - 25℃; Inert atmosphere; | 96% |
| With methanol at 20℃; for 0.833333h; | 87% |

| Conditions | Yield |
|---|---|
| With copper dichloride In methanol; water for 3h; Heating; | 95% |
| With Tris buffer; water; alpha cyclodextrin at 25℃; under 1500120 Torr; Rate constant; pH 8.3; further pressures; meta and para specific hydrolysis catalyzed by α-CD, pressure effects on, pressure dependence of log k, activation volume for the acylation, ΔV of CD-complex formation; |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; sodium hydride; N-methylaniline In diethyl ether; xylene at 120℃; for 6.5h; | 95% |
| With trimethylsilyl iodide at 105 - 114℃; for 0.25h; Microwave irradiation; Inert atmosphere; | 92% |
| With 1,3-dimethyl-2-imidazolidinone; sodium hexamethyldisilazane In tetrahydrofuran at 185℃; for 12h; further reagent: LDA; | 87% |

| Conditions | Yield |
|---|---|
| gold(0):poly(N-vinyl-2-pyrrolidine) nanocluster In phosphate buffer at 46.84℃; for 24h; pH=6.86; | A 95% B 4% |

| Conditions | Yield |
|---|---|
| With hydrogen; 0.75% Pd/Al2O3 at 180℃; Product distribution / selectivity; | 94.7% |
| With hydrogen at 180℃; Product distribution / selectivity; | 60.07% |
| With hydrogen; palladium on activated charcoal at 180℃; for 0 - 48h; Product distribution / selectivity; | 45.54% |
-
-
591-23-1
m-methylcyclohexanol

-
A
-
108-39-4
3-methyl-phenol

-
B
-
108-88-3
toluene

-
C
-
591-24-2, 625-96-7
3-Methylcyclohexanone

| Conditions | Yield |
|---|---|
| platinum; potassium oxide at 425℃; | A 94% B 1.8% C 4.2% |
| platinum; potassium oxide at 300℃; Product distribution; Kinetics; other content of catalyst, other temperature; |


| Conditions | Yield |
|---|---|
| Stage #1: 1-amino-3-methylbenzene With sulfuric acid at 20℃; Cooling with ice; Stage #2: With sodium nitrite In water Reflux; | 93% |
| Stage #1: 1-amino-3-methylbenzene With tetrafluoroboric acid In water at 20℃; for 0.0333333h; Stage #2: With sodium nitrite In water at 0℃; for 0.5h; Stage #3: With copper(I) oxide; copper(II) sulfate In water at 0 - 20℃; for 0.5h; | 53% |
| With phosphoric acid; water at 280℃; | |
| Diazotization; | |
| Stage #1: 1-amino-3-methylbenzene With isopentyl nitrite In N,N-dimethyl-formamide Flow reactor; Stage #2: With water In N,N-dimethyl-formamide Heating; Flow reactor; |
-
-
1847-82-1
Kohlensaeure-butyl-m-tolyl-ester

-
-
109-73-9
N-butylamine

-
A
-
13105-52-7
n-butylcarbamic acid n-butyl ester

-
B
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide | A 92% B n/a |

-
-
62790-75-4
1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-3-methylbenzene

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide at 20℃; for 3h; | 92% |
-
-
493035-82-8
(2-methyl-4-hydroxyphenyl)boronic acid

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With [bis(trifluoromethanesulfonyl)imidate](triphenylphosphine)gold(I); water In toluene at 90℃; for 1h; Microwave irradiation; Green chemistry; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: meta-bromotoluene With copper(l) iodide; 2-methyl-8-quinolinol; tetra(n-butyl)ammonium hydroxide In water; dimethyl sulfoxide at 130℃; for 14h; Stage #2: With hydrogenchloride In water; N,N-dimethyl-formamide at 20℃; | 91% |
| With copper(I) oxide; tetra(n-butyl)ammonium hydroxide; 1,10-phenanthroline-4,7-diol In water at 110℃; for 24h; Inert atmosphere; Schlenk technique; Sealed tube; Green chemistry; | 87% |
| With copper(l) iodide; potassium hydroxide In water at 120℃; for 8h; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; formic acid; N,N-dimethyl-formamide for 5h; Heating; | 90% |
| With sodium hydroxide; iron at 200℃; im geschlossenen Gefaess; |

| Conditions | Yield |
|---|---|
| Stage #1: 3-Iodotoluene With copper(l) iodide; tetra(n-butyl)ammonium hydroxide In water at 60℃; for 24h; Inert atmosphere; Sealed tube; Stage #2: With hydrogenchloride In water; ethyl acetate at 20℃; for 2h; Inert atmosphere; chemoselective reaction; | 89% |
| Stage #1: 3-Iodotoluene With copper(l) iodide; cesiumhydroxide monohydrate; 1,3-diphenylpropanedione In water; dimethyl sulfoxide at 130℃; for 24h; Inert atmosphere; Stage #2: With hydrogenchloride In dichloromethane; water; dimethyl sulfoxide at 20℃; Inert atmosphere; chemoselective reaction; | 84% |
| With tetra(n-butyl)ammonium hydroxide In water; dimethyl sulfoxide at 150℃; for 0.333333h; Flow reactor; | 84% |

| Conditions | Yield |
|---|---|
| With triethanolamine In water at 20℃; for 18h; Sonication; Irradiation; Green chemistry; | 89% |
| With methylene blue; N-ethyl-N,N-diisopropylamine In water; acetonitrile at 20℃; for 7h; Schlenk technique; Irradiation; |
-
-
74597-04-9
m-hydroxybenzyl bromide

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In 2-methyltetrahydrofuran at -15 - 20℃; for 24.16h; | 89% |

| Conditions | Yield |
|---|---|
| With Pd(2+)*2C2H6OS*2BF4(1-)*6C12H6N2O4(2-)*4Zr(4+)*4HO(1-)*4O(2-); oxygen In dimethyl sulfoxide at 100℃; under 760.051 Torr; for 35h; Sealed tube; Inert atmosphere; | 86% |
| With ethene; 5%-palladium/activated carbon In acetonitrile at 80℃; under 2280.15 Torr; for 24h; Autoclave; | 86% |
| With oxygen; 6C12H6N2O4(2-)*6Zr(4+)*4HO(1-)*4O(2-)*6Pd(2+)*12C2H6OS*12BF4(1-) In dimethyl sulfoxide at 100℃; for 35h; Inert atmosphere; | 86% |
-
-
102998-68-5
3-hydroxybenzyl iodide

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With indium; water for 5h; ultrasound; | 86% |

| Conditions | Yield |
|---|---|
| With oxygen; eosin Y disodium salt In acetonitrile for 20h; Sealed tube; Irradiation; | 86% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; choline chloride; urea; sodium hydroxide at 180℃; for 6h; Green chemistry; | 85.7% |

| Conditions | Yield |
|---|---|
| With bis(benzonitrile)palladium(II) dichloride In benzene for 20h; Heating; | 85% |
| With 12-TPA/SBA 15 In 1,4-dioxane at 110℃; | 72% |

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With oxalyl dichloride In 1,2-dichloro-ethane at 20℃; for 2.66667h; | 85% |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | 100% |
| at 20℃; for 0.666667h; | 100% |
| With Sulfate; titanium(IV) oxide In chloroform at 61℃; for 0.05h; | 99% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water for 0.00416667h; microwave irradiation; | 100% |
| With alkaline solution |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methyl-phenol With sodium hydride In N,N-dimethyl-formamide at 20℃; Stage #2: N,N-Dimethylcarbamoyl chloride In N,N-dimethyl-formamide at 20℃; for 0.5h; | 100% |
| Stage #1: 3-methyl-phenol With pyridine; potassium carbonate In acetonitrile for 0.333333h; Stage #2: N,N-Dimethylcarbamoyl chloride In acetonitrile for 2h; | 85% |
| With pyridine In benzene |
-
-
124-63-0
methanesulfonyl chloride

-
-
108-39-4
3-methyl-phenol

-
-
1077-02-7
3-methylphenyl methanesulfonate

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate at 0 - 20℃; for 0.166667h; Green chemistry; | 100% |
| With pyridine In dichloromethane at 0 - 20℃; Inert atmosphere; | 80.5% |
| With pyridine | |
| With triethylamine In dichloromethane at 0℃; |

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In dichloromethane for 1h; Ambient temperature; | 100% |
-
-
96-32-2
bromoacetic acid methyl ester

-
-
108-39-4
3-methyl-phenol

-
-
63051-20-7
m-tolyloxyacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In butanone for 5.5h; Heating; | 100% |
| With caesium carbonate In N,N-dimethyl-formamide at 25℃; for 4h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 24h; | 89% |
| With potassium carbonate In N-methyl-acetamide |
-
-
108-39-4
3-methyl-phenol

-
-
108791-52-2
<2H3>-m-cresol

| Conditions | Yield |
|---|---|
| With perchloric acid; d(4)-methanol at 75℃; for 144h; Inert atmosphere; | 100% |
| With water-d2; phosphorus tribromide for 5h; Heating; | 81% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 2h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; | |
| With potassium carbonate In dimethyl sulfoxide Heating; | |
| KF-Al2O3; 18-crown-6 ether In acetonitrile for 24h; Heating / reflux; | |
| With potassium carbonate In N,N-dimethyl-formamide for 8h; Inert atmosphere; Heating; |

| Conditions | Yield |
|---|---|
| With sodium carbonate; tris(triphenylphosphine)ruthenium(II) chloride at 140℃; for 4h; | 100% |
| With tris(triphenylphosphine)ruthenium(II) chloride; sodium carbonate at 120℃; for 4h; Inert atmosphere; regioselective reaction; | 86% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide; toluene at 20℃; for 7h; Inert atmosphere; | 91% |
| Stage #1: 3-methyl-phenol With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 0.5h; Inert atmosphere; Stage #2: propargyl bromide In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methyl-phenol With sodium hydride In tetrahydrofuran; mineral oil at 23℃; for 1h; Inert atmosphere; Stage #2: N,N-diethylcarbamyl chloride In tetrahydrofuran; mineral oil at 23℃; for 16h; Inert atmosphere; | 100% |
| Stage #1: 3-methyl-phenol With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.583333h; Stage #2: N,N-diethylcarbamyl chloride In tetrahydrofuran; mineral oil at 0 - 20℃; for 12.08h; | 85% |
| With potassium carbonate In acetonitrile at 85℃; for 18h; | 32% |
-
-
108-39-4
3-methyl-phenol

-
-
1523-15-5
2,4-dinitrophenyl benzoate

-
A
-
614-32-4
3-methylphenyl benzoate

-
B
-
14314-69-3
potassium 2,4-dinitrophenolate

| Conditions | Yield |
|---|---|
| With potassium hydrogencarbonate In N,N-dimethyl-formamide at 25℃; for 5h; | A 100% B n/a |

-
-
1365643-25-9
4'-methoxy-4-nitro-2,6-bis(trifluoromethyl)biphenyl

-
-
108-39-4
3-methyl-phenol

-
-
1365643-29-3
C22H16F6O2

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1-methyl-pyrrolidin-2-one at 90℃; for 31h; Inert atmosphere; | 100% |
-
-
69739-34-0
t-butyldimethylsiyl triflate

-
-
108-39-4
3-methyl-phenol

-
-
62790-75-4
1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-3-methylbenzene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |
-
-
80522-42-5
triisopropylsilyl trifluoromethanesulfonate

-
-
108-39-4
3-methyl-phenol

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In tetrahydrofuran at 50℃; for 16h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In butanone at 70 - 86℃; for 66h; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen In water at 20℃; under 7500.75 Torr; for 6h; Autoclave; | 99.7% |
| With nickel(II) oxide; hydrogen; palladium In hexane at 80℃; under 7500.75 Torr; for 10h; | 89% |
| With hydrogen; palladium on activated charcoal In hexane at 120℃; under 37503 Torr; Rate constant; var. solvents; |
-
-
108-39-4
3-methyl-phenol

-
-
612-13-5
2-cyanobenzyl chloride

-
-
951906-95-9
2-(3-methylphenoxymethyl)benzonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 110℃; for 6h; | 99.3% |
-
-
111-34-2
-butyl vinyl ether

-
-
108-39-4
3-methyl-phenol

-
-
93308-46-4
1-(n-butoxy)-1-(3-methylphenoxy)ethane

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 60 - 65℃; | 99% |
| With phosphoric acid at 20℃; |

| Conditions | Yield |
|---|---|
| With [μN,κP,κC,κN-{2-(i-Pr2PO),6-(CH2NBn)-(C6H3)}Ni]2 In benzene at 50℃; for 36h; | 99% |
| With sodium 3-methylphenoxide | |
| N-benzyl-trimethylammonium hydroxide Heating; | |
| With [{kp,kc,kp-2,6-(i-Pr2PO)2C6H3}Ni(NCMe)][OSO2CF3]; triethylamine In benzene-d6 at 20℃; for 4h; Michael condensation; | 100 %Spectr. |
| With [{kp,kc,kp-2,6-(i-Pr2PO)2C6H3}Ni(NCMe)][OSO2CF3]; triethylamine In toluene at 60℃; for 24h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water at 0 - 20℃; for 2h; Green chemistry; | 99% |
| With aluminum dodecatungstophosphate at 20℃; for 0.133333h; | 95% |
| In pyridine at 45℃; | 75% |

| Conditions | Yield |
|---|---|
| With benzyltrimethylammonium tribromide In methanol; dichloromethane | 99% |
| With bromine; acetic acid Ambient temperature; | 98% |
| With benzyltrimethylazanium tribroman-2-uide In methanol; dichloromethane for 1h; Ambient temperature; reagent 3.1 equivalent; | 93% |

| Conditions | Yield |
|---|---|
| With titanium superoxide; dihydrogen peroxide; acetic acid In water at 50℃; for 1h; | 99% |
| With dipyridinium dichromate In dichloromethane Ambient temperature; | 95% |
| With oxygen In water; acetonitrile at 40℃; under 15001.5 Torr; for 1h; Green chemistry; | 89% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 3h; | 99% |
| With sodium hydroxide; tetrabutylammomium bromide; potassium carbonate at 112℃; for 0.0833333h; Irradiation; | 95% |
| With sodium hydroxide; tetrabutylammomium bromide; potassium carbonate at 112℃; for 0.0833333h; microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With (R,R)-(salen)Co(H2O); 3 A molecular sieve In various solvent(s) at 25℃; for 16h; | 99% |
| With 1,1,1,3,3,3-hexafluoro-2-(trifluoromethyl)propan-2-ol; polysytrene-immobilized chiral Co-salen complex In tetrahydrofuran at 20℃; for 24h; | 96% |
| In acetonitrile at 23℃; for 14h; enantioselective reaction; | 95% |
| With Co(salen) macrocycles 1(OTs) In tert-butyl methyl ether at 20℃; for 19h; optical yield given as %ee; enantioselective reaction; | 91% |
m-Cresol Specification
The m-Cresol is an organic compound with the formula C7H8O. The IUPAC name of this chemical is 3-methylphenol. With the CAS registry number 108-39-4, it is also named as 1-hydroxy-3-methylbenzene. The product's category is Phenoles and Thiophenoles. Besides, it is colourless to light yellow liquid, which should be stored in a cool and ventilated place. It is used as a color film of the dye intermediates and for the production of fenitrothion, fenthion, metolcarb, permethrin and other pesticides. It is mainly used in pesticide, medicine, spices, resin, plasticizer, film and antioxidant.
Physical properties about m-Cresol are: (1)ACD/LogP: 2.043; (2)ACD/LogD (pH 5.5): 2.04; (3)ACD/LogD (pH 7.4): 2.04; (4)ACD/BCF (pH 5.5): 21.03 ; (5)ACD/BCF (pH 7.4): 20.98; (6)ACD/KOC (pH 5.5): 307.93; (7)ACD/KOC (pH 7.4): 307.28; (8)#H bond acceptors: 1 ; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 1; (11)Index of Refraction: 1.546; (12)Molar Refractivity: 32.959 cm3; (13)Molar Volume: 104.139 cm3; (14)Polarizability: 13.066 10-24cm3; (15)Surface Tension: 38.814998626709 dyne/cm ; (16)Density: 1.038 g/cm3; (17)Flash Point: 86.111 °C; (18)Enthalpy of Vaporization: 45.641 kJ/mol; (19)Boiling Point: 202.279 °C at 760 mmHg; (20)Vapour Pressure: 0.207000002264977 mmHg at 25°C
Preparation of m-Cresol: this chemical can be prepared by Bisabolangelon. This reaction will need reagent NH2OH*HCl, Na2CO3 and solvent ethanol, H2O. The reaction temperature is 50 °C. The yield is about 49%.
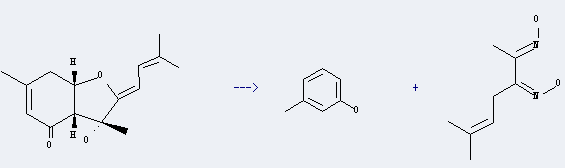
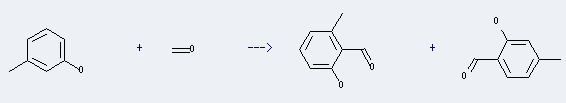
Uses of m-Cresol: it can be used to produce 2-hydroxy-6-methyl-benzaldehyde by heating. It will need reagent MgCl2, Et3N and solvent acetonitrile with reaction time of 4 hours. The yield is about 12%.
When you are using this chemical, please be cautious about it as the following:
It is harmful by inhalation, in contact with skin and if swallowed. Besides, this chemical can cause burns and danger of very serious irreversible effects through inhalation and in contact with skin. When you are using it, wear suitable gloves and eye/face protection. In case of accident or if you feel unwell seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)InChI=1S/C7H8O/c1-6-3-2-4-7(8)5-6/h2-5,8H,1H3;
(2)InChIKey=RLSSMJSEOOYNOY-UHFFFAOYSA-N;
(3)Smilesc1c(cccc1O)C;
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | subcutaneous | 180mg/kg (180mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 80, Pg. 233, 1944. | |
| dog | LDLo | intravenous | 150mg/kg (150mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 5, Pg. 56, 1959. | |
| frog | LDLo | subcutaneous | 250mg/kg (250mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1361, 1935. | |
| guinea pig | LDLo | intraperitoneal | 100mg/kg (100mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1361, 1935. | |
| guinea pig | LDLo | subcutaneous | 300mg/kg (300mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 5, Pg. 56, 1959. | |
| mouse | LD50 | intraperitoneal | 168mg/kg (168mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 5, Pg. 56, 1959. | |
| mouse | LD50 | oral | 828mg/kg (828mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 18(2), Pg. 58, 1974. | |
| mouse | LD50 | unreported | 600mg/kg (600mg/kg) | Gigiena Truda i Professional'naya Patologiya v Estonskoi SSR. Labor Hygiene and Occupational Pathology in the Estonian SSR. Vol. 8, Pg. 145, 1972. | |
| mouse | LDLo | subcutaneous | 450mg/kg (450mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1361, 1935. | |
| rabbit | LD50 | skin | 2050mg/kg (2050mg/kg) | GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE | BIOFAX Industrial Bio-Test Laboratories, Inc., Data Sheets. Vol. 3-5/1969, |
| rabbit | LDLo | intravenous | 280mg/kg (280mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 80, Pg. 233, 1944. | |
| rabbit | LDLo | oral | 1400mg/kg (1400mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 80, Pg. 233, 1944. | |
| rabbit | LDLo | subcutaneous | 500mg/kg (500mg/kg) | "Abdernalden's Handbuch der Biologischen Arbeitsmethoden." Vol. 4, Pg. 1361, 1935. | |
| rat | LC50 | inhalation | > 710mg/m3/1H (710mg/m3) | BIOFAX Industrial Bio-Test Laboratories, Inc., Data Sheets. Vol. 3-5/1969, | |
| rat | LD50 | oral | 242mg/kg (242mg/kg) | GASTROINTESTINAL: PERITONITIS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | BIOFAX Industrial Bio-Test Laboratories, Inc., Data Sheets. Vol. 3-5/1969, |
| rat | LD50 | skin | 1100mg/kg (1100mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 18(2), Pg. 58, 1974. | |
| rat | LDLo | subcutaneous | 900mg/kg (900mg/kg) | "Handbook of Toxicology," 4 vols., Philadelphia, W.B. Saunders Co., 1956-59Vol. 5, Pg. 56, 1959. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View