-
Name
2-Acetylbutyrolactone
- EINECS 208-235-2
- CAS No. 517-23-7
- Article Data32
- CAS DataBase
- Density 1.191 g/cm3
- Solubility 310 g/L (20 °C) in water
- Melting Point <25 °C
- Formula C6H8O3
- Boiling Point 253.078 °C at 760 mmHg
- Molecular Weight 128.128
- Flash Point 128.244 °C
- Transport Information
- Appearance Colorless to light yellow liquid
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Acetoaceticacid, 2-(2-hydroxyethyl)-, g-lactone (6CI,7CI);2-Acetyl-g-butyrolactone;2-Acetylbutanolide;2-Oxo-3-acetyltetrahydrofuran;3-Acetyl-4,5-dihydro-2(3H)-furanone;3-Acetyl-4,5-dihydrofuran-2-one;3-Acetyldihydro-2(3H)-furanone;3-Acetyldihydrofuran-2-one;3-Acetyltetrahydro-2-furanone;Dihydro-3-acetyl-2(3H)-furanone;NSC 2019;a-(2-Hydroxyethyl)acetoacetic acidg-lactone;a-Acetobutyrolactone;a-Acetyl-g-butyrolactone;a-Acetyl-g-hydroxybutyric acid g-lactone;a-Acetylbutyrolactone;
- PSA 43.37000
- LogP 0.13850
Synthetic route

| Conditions | Yield |
|---|---|
| With ethyl hydrazine hydrochloride In methanol at 10 - 20℃; for 2h; Temperature; | A 98.9% B 98.29% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-butanolide; acetic acid methyl ester With sodium methylate In methanol at 45 - 90℃; under 750.075 Torr; for 10h; Inert atmosphere; Large scale; Stage #2: With carbon dioxide In methanol; water at 0 - 35℃; under 6000.6 Torr; for 0.5h; Temperature; Pressure; Reagent/catalyst; Large scale; | 96% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate at 75℃; for 10h; Reflux; | 93% |
| Stage #1: 4-butanolide; ethyl acetate With sodium methylate at 50 - 100℃; for 5h; Autoclave; Stage #2: With sulfuric acid at -5 - 5℃; for 5h; pH=6 - 7; | 83% |
| With sodium at 80℃; for 8h; Mechanism; |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethyl acetate at 60 - 95℃; under 750.075 - 7500.75 Torr; for 3.5h; Solvent; Temperature; Reagent/catalyst; Autoclave; | 79.31% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at -5 - 0℃; for 50h; | 78% |
| With triethylamine In methanol at 5 - 95℃; under 1500.15 - 2250.23 Torr; for 2.15 - 5h; Product distribution / selectivity; | 40.4% |
| With N,N,N',N'-tetramethylguanidine In methanol at 5 - 65℃; under 1500.15 - 2250.23 Torr; for 1.15 - 6.18333h; Product distribution / selectivity; | 30.8% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-butanolide With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 0.25h; Inert atmosphere; Stage #2: acetic anhydride In tetrahydrofuran at -78℃; for 1h; Inert atmosphere; | 42% |
-
-
75-21-8
oxirane

-
-
64-17-5
ethanol

-
-
1007476-32-5
sodium ethyl acetylacetate enolate

-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
75-21-8
oxirane

-
-
1007476-32-5
sodium ethyl acetylacetate enolate

-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

| Conditions | Yield |
|---|---|
| With ethanol |
-
-
64-17-5
ethanol

-
-
1007476-32-5
sodium ethyl acetylacetate enolate

-
-
107-07-3
2-chloro-ethanol

-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran


| Conditions | Yield |
|---|---|
| With sodium hydroxide 1.) water, EtOH, 0 deg C, 30 min, 2.) 0 - 5 deg C, 72 h; Yield given. Multistep reaction; | |
| With sodium hydroxide; sulfuric acid In water; ethylene glycol |
-
-
96-48-0
4-butanolide

-
-
141-52-6
sodium ethanolate

-
-
141-78-6
ethyl acetate

-
A
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
B
-
1071-73-4
5-Hydroxy-2-pentanone

-
C
-
999-10-0
ethyl 4-hydroxybutanoate

-
D
-
25560-91-2
ethyl 4-(acetyloxy)butanoate

-
E
-
141-97-9
ethyl acetoacetate

-
F
-
65652-24-6
α-(2-Tetrahydrofuranylidene)-γ-butyrolactone

| Conditions | Yield |
|---|---|
| at 100℃; under 1064 Torr; for 0.5h; Product distribution; various molar ratio of the reagents, other temp., other pressure, other time; |


| Conditions | Yield |
|---|---|
| With 18-crown-6 ether 1.) THF, RT, 15 min, 2.) THF, RT, 3 h; Yield given. Multistep reaction; |
-
-
64620-59-3
3-<1-(N-phenylamino)-ethylidene>-4,5-dihydro-2(3H)-furanone

-
A
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
B
-
62-53-3
aniline

| Conditions | Yield |
|---|---|
| With water at 25℃; Rate constant; Mechanism; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; Trioxane In methanol Dehydration; Heating; |

| Conditions | Yield |
|---|---|
| With sodium |

-
-
7647-01-0
hydrogenchloride

-
-
412012-07-8
2-acetyl-2-(methylsulfanyl-methyl)-succinic acid diethyl ester

-
A
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
B
-
89533-75-5
3-methylene-4-oxopentanoic acid

| Conditions | Yield |
|---|---|
| 10 h Erhitzen.; |


| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78℃; |
-
-
96-48-0
4-butanolide

-
-
75-07-0
acetaldehyde

-
-
141-78-6
ethyl acetate

-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

| Conditions | Yield |
|---|---|
| With sodium carbonate at 44 - 80℃; for 8.66667h; Temperature; Autoclave; |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; indium In methanol; water for 10h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; indium In methanol; water for 10h; Ambient temperature; | 100% |
| With tin In hydrogenchloride; methanol for 20h; Product distribution; Ambient temperature; other reagent, solvent, reaction time; also with allyl bromide; | 90% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
75-77-4
chloro-trimethyl-silane

-
-
133400-76-7
4-<1-(trimethylsiloxy)ethenyl>-5-(trimethylsiloxy)-2,3-dihydrofuran

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78 - 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With chlorine at -10℃; for 5h; Temperature; Saturated gas; | 99.3% |
| With sulfuryl dichloride at 5 - 10℃; for 3h; | 99.1% |
| With chlorine In dichloromethane at -10℃; for 5h; Temperature; Reagent/catalyst; Flow reactor; | 99% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
135366-64-2
α-acetyl-α-hydroxy-γ-butyrolactone

| Conditions | Yield |
|---|---|
| With 3,3-dimethyldioxirane; nickel diacetate In water; acetone for 3.5h; Ambient temperature; | 99% |
| With 3,3-dimethyldioxirane In acetone at 20℃; for 72h; | 98% |
| With samarium (III) iodide; iodine In tetrahydrofuran; water at 25℃; for 12h; Catalytic behavior; Green chemistry; | 95% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
78-94-4
methyl vinyl ketone

-
-
58623-81-7, 74627-02-4, 176101-57-8
3-acetyl-3-(3-oxobutyl)-4,5-dihydrofuran-2(3H)-one

| Conditions | Yield |
|---|---|
| With silica gel In neat (no solvent) at 0 - 90℃; for 20h; Michael Addition; | 99% |
| NDEAP grafted on silica In water at 80℃; for 0.233333h; Michael addition; microwave irradiation; | 84% |
| With iron(III) chloride In dichloromethane at 20℃; Michael reaction; | 36% |

| Conditions | Yield |
|---|---|
| With silica gel at 20℃; for 2h; | 99% |
| With β‐cyclodextrin In water at 20℃; for 1.5h; chemospecific reaction; | 96% |
| With cerium(III) chloride; tetrabutylammomium bromide at 20℃; for 0.0833333h; | 95% |

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -78 - 20℃; | 99% |
| With triethylamine In benzene at 20℃; for 14h; Etherification; |

| Conditions | Yield |
|---|---|
| With silica gel at 20℃; for 3.5h; | 99% |
| at 23℃; for 4h; | 89% |

| Conditions | Yield |
|---|---|
| With silica gel at 20℃; for 4h; | 99% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

| Conditions | Yield |
|---|---|
| With ammonia; silica gel at 20℃; for 5h; | 99% |
| With ammonium acetate; tetraethoxy orthosilicate In ethanol Heating; | 98% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In toluene at 25℃; for 16h; | 99% |
| With boron trifluoride diethyl etherate In toluene at 25℃; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With 1-[3,5-bis(trifluoromethyl)phenyl]-3-[(2S)-dimethylamino-(1S)-phenylpropyl]thiourea In diethyl ether at 0℃; for 17h; nitro-Michael reaction; enantioselective reaction; | 99% |
| With 3-((3,5-bis(trifluoromethyl)benzyl)amino)-4-((2-(dimethylamino)ethyl)-amino)cyclobut-3-ene-1,2-dione In toluene at 25℃; for 24h; Michael Addition; Inert atmosphere; | 87% |
| (3ξ,8α,9R)-cinchonan-6',9-diol In tetrahydrofuran at 20℃; for 0.5h; Product distribution / selectivity; Michael Condensation; | |
| With 1,4-diaza-bicyclo[2.2.2]octane In dichloromethane at 20℃; Michael Addition; | |
| With 1,4-diaza-bicyclo[2.2.2]octane In neat (no solvent) at 20℃; Michael Addition; |

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 24h; | 99% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

| Conditions | Yield |
|---|---|
| With chlorine at 25 - 45℃; under 760.051 - 1520.1 Torr; for 2h; Temperature; Pressure; Concentration; | 98.6% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
101561-12-0
C12H12N2O3

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 0.1h; Ambient temperature; | 98% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
155321-91-8
(-)-syn-(3R,1'R)-α-(hydroxyethyl)-γ-butyrolactone

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide under 375030 Torr; Ambient temperature; Saccharomyces cerevisiae ML29; | 98% |
| In N,N-dimethyl-formamide at 26℃; for 24h; Yarrowia lipolytica Y5; | 95% |
| With nicotinic acid; ammonium sulfate; potassium dihydrogenphosphate; 5-hydroxy-6-methyl-3,4-pyridinedimethanol; D-glucose; D-myo-inositol; vitamin B1; boric acid; calcium (R)-pantothenate; magnesium sulfate; copper(II) sulfate; zinc(II) sulfate; potassium iodide; calcium chloride; manganese(ll) chloride; iron(II) chloride; biotin In ethanol; water at 29℃; for 96h; Yarrowia lipolytica PFL9CE; | 69% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
292638-85-8
acrylic acid methyl ester

-
-
280568-02-7
3-acetyl-2-oxotetrahydrofuran-3-propanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With polystyrene supported P1 iminophosphorane P-BEMP In tetrahydrofuran at 20℃; for 3h; Michael addition; | 98% |
| With sodium hydride In tert-butyl alcohol at 35℃; for 4h; Michael addition; | 96% |
| With 1,4-diaza-bicyclo[2.2.2]octane; water at 20℃; Michael addition; | 95% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
106-47-8
4-chloro-aniline

-
-
64620-30-0
3-[(Z)-1-(4-chloro-anilino)-ethylidene]-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| With cerium(III) chloride; tetrabutylammomium bromide at 20℃; for 0.0833333h; | 98% |
| With β‐cyclodextrin In water at 20℃; for 0.333333h; chemospecific reaction; | 95% |
| With tris(trifluoroacetato)bismuth(III); tetrabutylammomium bromide at 20℃; for 0.0833333h; | 93% |
| cerium(III) chloride In water at 20℃; for 0.0833333h; | 88% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 12h; Heating; | 98% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
41601-44-9
3-amino-4,6-dimethylpyrazolo[3,4-b]pyridine

-
-
1220997-01-2
3-(2-hydroxyethyl)-2,8,10-trimethylpyrido[2',3':3,4]pyrazolo[1,5-a]pyrimidin-4-ol

| Conditions | Yield |
|---|---|
| In diphenylether at 220 - 225℃; for 0.25h; | 98% |

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; bis[2-(diphenylphosphino)phenyl] ether; ytterbium(III) triflate In 1,2-dichloro-ethane at 70℃; for 48h; Schlenk technique; Inert atmosphere; Glovebox; Sealed tube; diastereoselective reaction; | A 98% B n/a |


| Conditions | Yield |
|---|---|
| With sodium iodide at 192℃; under 4500.45 Torr; Reagent/catalyst; Temperature; Pressure; | 97.12% |
| Stage #1: 3-acetyl-2-oxo-4,5-dihydrofuran With hydrogenchloride In water at 100℃; for 2h; Stage #2: With sodium hydroxide In water at 80 - 100℃; for 2h; | 90.2% |
| With 1-methyl-pyrrolidin-2-one; sodium iodide at 170℃; for 3h; | 59% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran Ambient temperature; | 96% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at -78 - -50℃; for 2h; domino Michael-O-alkylation; | 96% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
106-49-0
p-toluidine

-
-
64620-32-2
3-[(Z)-1-(4-toluidino)ethylidene]-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| With β‐cyclodextrin In water at 20℃; for 1h; chemospecific reaction; | 96% |
| ammonium cerium(IV) nitrate at 20℃; for 1.16667h; | 95% |
| zirconium(IV) chloride at 20℃; for 0.5h; | 92% |

| Conditions | Yield |
|---|---|
| With tris(trifluoroacetato)bismuth(III); tetrabutylammomium bromide at 20℃; for 0.166667h; | 96% |
| With β‐cyclodextrin In water at 20℃; for 1.33333h; chemospecific reaction; | 95% |
| With tris(trifluoroacetato)bismuth(III) In water at 20℃; for 10h; | 93% |
| With cerium(III) chloride; tetrabutylammomium bromide at 20℃; for 0.0833333h; | 92% |
| cerium(III) chloride In water at 20℃; for 0.166667h; | 85% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
65185-68-4
(E)-2-bromo-β-nitrostyrene

| Conditions | Yield |
|---|---|
| With 3-((3,5-bis(trifluoromethyl)benzyl)amino)-4-((2-(dimethylamino)ethyl)-amino)cyclobut-3-ene-1,2-dione In toluene at 25℃; for 24h; Michael Addition; Inert atmosphere; | 96% |
-
-
517-23-7
3-acetyl-2-oxo-4,5-dihydrofuran

-
-
74-88-4
methyl iodide

-
-
1123-19-9, 112607-28-0, 112607-29-1
3-acetyl-3-methyl-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 50℃; for 16h; Large scale; | 95% |
| Stage #1: 3-acetyl-2-oxo-4,5-dihydrofuran With sodium methylate In benzene Stage #2: methyl iodide Further stages.; | 83% |
| With potassium carbonate In acetone | 80% |
2-Acetylbutyrolactone Specification
The 2-Acetylbutyrolactone, with the CAS registry number 517-23-7, is also known as 3-Acetyldihydro-2(3H)-furanone. It belongs to the product categories of Ketone; Furan & Benzofuran; Carbonyl Compounds; Lactones; Organic Building Blocks; Heterocycles; Intermediates & Fine Chemicals; Pharmaceuticals. Its EINECS registry number is 208-235-2. This chemical's molecular formula is C6H8O3 and molecular weight is 128.13. What's more, its IUPAC name is called 3-Acetyloxolan-2-one. It should be stored in a cool, dry and sealed place.
Physical properties about 2-Acetylbutyrolactone are: (1)ACD/LogP: -1.217; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.22; (4)ACD/LogD (pH 7.4): -1.22; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 5.19; (8)ACD/KOC (pH 7.4): 5.19; (9)#H bond acceptors: 3; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 43.37 Å2; (13)Index of Refraction: 1.459; (14)Molar Refractivity: 29.388 cm3; (15)Molar Volume: 107.507 cm3; (16)Polarizability: 11.65×10-24cm3; (17)Surface Tension: 39.39 dyne/cm; (18)Density: 1.192 g/cm3; (19)Flash Point: 128.244 °C; (20)Enthalpy of Vaporization: 49.045 kJ/mol; (21)Boiling Point: 253.078 °C at 760 mmHg; (22)Vapour Pressure: 0.019 mmHg at 25 °C.
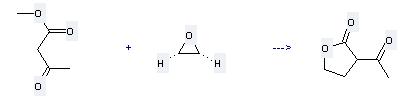
Preparation of 2-Acetylbutyrolactone: this chemical can be prepared by oxirane with acetoacetic acid methyl ester. This reaction needs reagent sodium hydroxide and solvents H2O, ethanol at temperature of -5 – 0 °C. The reaction time is 50 hours. The yield is 78 %.
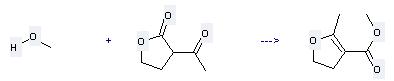
Uses of 2-Acetylbutyrolactone: (1) it is an important intermediate to synthesize vitamin B,3,4 - disubstituted pyridine and 5-(β-hydroxyethyl) -4- methyl-thiazole. (2) it is used to produce other chemicals. For example, it can react with methanol to get 2-methyl-4,5-dihydro-furan-3-carboxylic acid methyl ester. The reaction occurs with reagent hydrogen chloride and other condition of heating for 4 days. The yield is 19 %.
When you are dealing with this chemical, you should be very careful. This chemical is inflammation to the skin, eyes and respiratory system or other mucous membranes. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O=C1OCCC1C(=O)C
(2) InChI: InChI=1S/C6H8O3/c1-4(7)5-2-3-9-6(5)8/h5H,2-3H2,1H3
(3) InChIKey: OMQHDIHZSDEIFH-UHFFFAOYSA-N
Related Products
- 2-Acetylbutyrolactone
- 2-Acetylbutyrolactone-3,3,4,4-d4
- 51725-82-7
- 51726-83-1
- 517-28-2
- 51732-34-4
- 5173-46-6
- 5173-65-9
- 51-74-1
- 51742-11-1
- 51742-42-8
- 5174-32-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View