-
Name
4-Chlorophenylacetone
- EINECS 226-986-4
- CAS No. 5586-88-9
- Article Data72
- CAS DataBase
- Density 1.14 g/cm3
- Solubility
- Melting Point 6-8 °C
- Formula C9H9ClO
- Boiling Point 236.5 °C at 760 mmHg
- Molecular Weight 168.623
- Flash Point 116.3 °C
- Transport Information
- Appearance Clear light yellow liquid
- Safety 24/25
- Risk Codes 22-52
-
Molecular Structure
- Hazard Symbols Xn
- Synonyms 2-Propanone,(p-chlorophenyl)- (6CI,7CI);2-Propanone, 1-(p-chlorophenyl)- (8CI);p-Chlorophenyl-2-propanone;(p-Chlorophenyl)acetone;1-(4-Chlorophenyl)acetone;1-(4-Chlorophenyl)propan-2-one;1-(p-Chlorophenyl)-2-propanone;4-Chlorobenzylmethyl ketone;Methyl p-chlorobenzyl ketone;NSC 22985;p-Chlorobenzyl methylketone;
- PSA 17.07000
- LogP 2.47150
Synthetic route
-
-
820-71-3
methallyl acetate

-
-
1073-69-4
N-4-chlorophenylhydrazine

-
A
-
1621652-06-9
3-(4-chlorophenyl)-2-hydroperoxy-2-methylpropyl acetate

-
B
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; acetic acid In acetonitrile at 23℃; for 1h; | A n/a B 100% |

-
-
1073-69-4
N-4-chlorophenylhydrazine

-
-
126-98-7
methacrylonitrile

-
A
-
1621652-17-2
3-(4-chlorophenyl)-2-hydroperoxy-2-methylpropanenitrile

-
B
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; acetic acid In acetonitrile at 23℃; for 1h; | A n/a B 100% |

-
-
1163136-86-4
1-chloro-4-(2-nitro-1(Z)-propenyl)benzene

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With formaldehyd In 1,4-dioxane; perchloric acid; dichloromethane for 0.333333h; Ambient temperature; | 93% |
| With formaldehyd In perchloric acid; dichloromethane for 0.333333h; Product distribution; Ambient temperature; chemical and electrochemical reduction; further nitro alkenes, further reagents; | 93% |
| With aluminium; nickel dichloride In tetrahydrofuran Substitution; | 89% |
| With sodium hypophosphite; nickel In ethanol; acetate buffer; water at 60℃; for 3h; pH=5; | 47% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-chloro-4-(1-methylethenyl)-benzene With ammonium iodide; water; sodium dodecyl-sulfate for 0.5h; Stage #2: With Oxone at 20℃; for 7h; regioselective reaction; | 86% |
| With [hydroxy(tosyloxy)iodo]benzene In methanol at 20℃; for 1h; | 80% |
| With Oxone; iodine In 1,2-dimethoxyethane; water at 20℃; for 3h; regioselective reaction; | 80% |
-
-
1021934-07-5
C9H10BrClO

-
A
-
6285-05-8
4'-chloropropiophenone

-
B
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With diethylzinc In dichloromethane at 20℃; for 2h; | A 5% B 86% |

| Conditions | Yield |
|---|---|
| With ethyl nitrite; potassium carbonate In acetone at 0 - 20℃; for 3.5h; | 80.2% |
| With tert.-butylnitrite; water; salicylic acid In acetonitrile at 20℃; for 3h; Inert atmosphere; Schlenk technique; | 62% |
| With hydrogenchloride; tin; tin(ll) chloride; sodium nitrite In water; N,N-dimethyl-formamide; acetone at 0 - 20℃; Reagent/catalyst; Meerwein Arylation; | 51% |
-
-
98236-14-7
1-Chloro-4-(2-iodo-1-methoxy-1-methyl-ethyl)-benzene

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; for 1h; | 80% |
-
-
1198184-09-6
4-chlorophenyl 1H-imidazole-1-sulfonate

-
-
67-64-1
acetone

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 60℃; for 24h; Inert atmosphere; chemoselective reaction; | 79% |
-
-
108-22-5
Isopropenyl acetate

-
-
673-41-6
p-chlorobenzenediazonium tetrafluoroborate

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With tetrakis(pentafluorophenyl)porphyrin In nitromethane; N,N-dimethyl-formamide at 0℃; for 2h; Sealed tube; Inert atmosphere; Darkness; Irradiation; | 76% |
| With potassium acetate In water; acetone at 20℃; | 70% |
-
-
50337-50-3
2-(4'-chlorophenyl)-3-methyloxirane

-
B
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-(4'-chlorophenyl)-3-methyloxirane With boron trifluoride diethyl etherate In dichloromethane at -20℃; for 0.166667h; Stage #2: With water; sodium hydrogencarbonate In dichloromethane optical yield given as %de; stereoselective reaction; | A 76% B 6% |

| Conditions | Yield |
|---|---|
| With tributyltin methoxide; dichlorobis(tri-O-tolylphosphine)palladium In toluene at 100℃; for 5h; | 73% |
-
-
106-39-8
bromochlorobenzene

-
-
108-22-5
Isopropenyl acetate

-
A
-
79-20-9
acetic acid methyl ester

-
B
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| dichlorobis(tri-O-tolylphosphine)palladium In toluene at 100℃; for 5h; | A n/a B 73% |


-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| Sample heated for 1.0 h at 200-220°C and 1 torr.; Sublimation; chromy. (F-20 alumina, CCl4/CHCl3).; | 73% |
-
-
37629-52-0
(E)-1-(4-chlorophenyl)-2-nitropropene

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid; zinc In N,N-dimethyl-formamide for 0.166667h; | 70% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium phosphate tribasic trihydrate In dimethyl sulfoxide at 90℃; for 20h; Inert atmosphere; | 67% |
| With copper(l) iodide; potassium phosphate tribasic trihydrate In dimethyl sulfoxide at 90℃; for 12h; Inert atmosphere; |
-
-
29125-75-5
p-chloro(methylstyrene)

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With chloropyridinecobaloxime(III); water; 9-(2-mesityl)-10-methylacridinium perchlorate In acetonitrile at 20℃; for 24h; Wacker Oxidation; Inert atmosphere; Schlenk technique; Irradiation; regioselective reaction; | 62% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium phosphate tribasic trihydrate In dimethyl sulfoxide at 110℃; for 20h; Inert atmosphere; | 61% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorophenylacetic Acid With isopropylmagnesium chloride In tetrahydrofuran; tert-butyl methyl ether at -10 - 25℃; for 1.5h; Inert atmosphere; Schlenk technique; Stage #2: ethyl acetate In tetrahydrofuran; tert-butyl methyl ether at 0 - 5℃; for 1.25h; Inert atmosphere; Schlenk technique; | 61% |
-
-
50337-50-3
2-(4'-chlorophenyl)-3-methyloxirane

-
B
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Inert atmosphere; | A 57% B 27% |

-
-
108-24-7
acetic anhydride

-
-
104-83-6
1-Chloro-4-(chloromethyl)benzene

-
A
-
5406-33-7
4-chlorobenzyl acetate

-
B
-
138376-43-9
(E)-1-(4-chlorophenyl)prop-1-en-2-yl acetate

-
C
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With tetraethylammonium tosylate In N,N-dimethyl-formamide Ambient temperature; electroreduction: Pb-cathode, carbon-rod anode, 15mA/cm-2; | A 8% B 56% C 7% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; iron; iron(II) chloride In ethanol; water at 70℃; for 6.5h; | 49% |
| With hydrogenchloride; iron; iron(II) chloride In ethanol | |
| With hydrogenchloride; iron(III) chloride; iron | |
| With hydrogenchloride; iron(III) chloride; iron for 1h; Heating; | |
| With hydrogenchloride; iron |
-
-
710-20-3
1-chloro-4-(2-nitropropenyl)benzene

-
A
-
1454-65-5
1-(4-chlorophenyl)propan-2-one oxime

-
B
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With sulfuric acid; toluene-4-sulfonic acid In methanol at 0 - 5℃; ceramic diaphragm, carbon-rod anode, platinum cathode, 4.5 F/mol, 0.1A, cathode potential -1.45 to 1.65V vs. SCE; | A 45% B 8% |
-
-
19350-69-7
4-chlorobenzaldehyde p-toluenesulfonylhydrazone

-
-
75-20-7
calcium carbide

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; water; copper(l) chloride In dimethyl sulfoxide at 110℃; for 8h; | 9% |
-
-
1878-66-6
4-chlorophenylacetic Acid

-
-
108-24-7
acetic anhydride

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With sodium acetate | |
| With pyridine modified Dakin West reaction; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 100℃; anschliessendes Erhitzen auf 100grad; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; water | |
| With sulfuric acid; water Yield given; |

| Conditions | Yield |
|---|---|
| In diethyl ether | |
| In diethyl ether at 0℃; |
-
-
30186-24-4
ethyl 2-(4-chlorophenyl)-3-oxobutanoate

-
A
-
99-91-2
para-chloroacetophenone

-
B
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| In water at 230℃; Title compound not separated from byproducts; | A 23 % Chromat. B 77 % Chromat. |
-
-
29865-54-1
1-(4'-chlorophenyl)-2-nitropropane

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; 1,8-diazabicyclo[5.4.0]undec-7-ene; 3-chloro-benzenecarboperoxoic acid 1.) dichloromethane, 30 min, 0 degC; 2.) dichloromethane, 30 min, room temperature; Yield given. Multistep reaction; |
-
-
144380-82-5
(Z)-1-(4-Chloro-phenyl)-propen-2-ol

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| In benzene-d6 at 25℃; Equilibrium constant; other solvent; |
-
-
107-87-9
2-Pentanone

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
A
-
625-30-9, 33985-20-5, 54542-13-1, 63493-28-7
(R)‐2‐aminopentane

| Conditions | Yield |
|---|---|
| With Candida boidinii formate dehydrogenase; pyridoxal 5'-phosphate; Aspergillus terreus ω-trans aminase; Lysinibacillus fusiformis leucine dehydrogenase; ammonium formate; nicotinamide adenine dinucleotide In aq. buffer at 30℃; for 24h; pH=8.8; Catalytic behavior; Green chemistry; Enzymatic reaction; | A 99.3% B 99.1% |

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With Candida boidinii formate dehydrogenase; pyridoxal 5'-phosphate; ammonium formate; nicotinamide adenine dinucleotide In aq. buffer at 30℃; for 24h; pH=8.8; Green chemistry; Enzymatic reaction; | 99.1% |
| With pyridoxal 5'-phosphate; amine transaminases from Aspergillus fumigatus; isopropylamine In N,N-dimethyl-formamide at 30℃; pH=7.5 - 8; Enzymatic reaction; enantioselective reaction; | n/a |
| With ammonium hydroxide; ammonium acetate; GkAmDH In water at 40℃; for 24h; pH=9; Enzymatic reaction; | n/a |
-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
593960-71-5
2-chloro-5-(2-oxo-propyl)-benzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid at -10 - 20℃; for 36h; | 98% |
| With chlorosulfonic acid at -10 - 20℃; | |
| With chlorosulfonic acid at -10 - 20℃; for 10h; | |
| With chlorosulfonic acid at 40℃; for 2h; Cooling with ice; |
-
-
7790-94-5
chlorosulfonic acid

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
593960-71-5
2-chloro-5-(2-oxo-propyl)-benzenesulfonyl chloride

| Conditions | Yield |
|---|---|
| at -10 - 20℃; for 36h; | 98% |
| at -10 - 20℃; for 18h; | 65% |
-
-
109-77-3
malononitrile

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
69358-83-4
2-(1-(4-chlorophenyl)propan-2-ylidene)malononitrile

| Conditions | Yield |
|---|---|
| With ammonium acetate; acetic acid In benzene at 120℃; Knoevenagel Condensation; Dean-Stark; | 96% |
| With ammonium acetate In acetic acid; benzene |
-
-
95-92-1
oxalic acid diethyl ester

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
595610-34-7
5-(4-chlorophenyl)-2,4-dioxopentanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In toluene at 0 - 20℃; for 18.25h; | 96% |
| With ethanol; sodium hydride at 20℃; for 16h; | 74% |
| With sodium ethanolate In ethanol at 20℃; for 18h; Claisen condensation; |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
2510-74-9, 5121-74-4, 1657-56-3, 144606-14-4
(E)-1,2-di(4-chlorophenyl)ethene

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; | 96% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dimethyl sulfoxide at 25℃; for 0.5h; regioselective reaction; | 95% |
| With tetra(n-butyl)ammonium hydrogensulfate; potassium hydroxide In water at 100℃; for 24h; Inert atmosphere; regioselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dimethyl sulfoxide at 25℃; for 0.75h; regioselective reaction; | 92% |
-
-
447-31-4
Desyl chloride

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
1333377-89-1
3-(4-chlorophenyl)-1,2-diphenylpentane-1,4-dione

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-chlorophenyl)propan-2-one With sodium hexamethyldisilazane In tetrahydrofuran at 0℃; Inert atmosphere; Stage #2: With zinc(II) chloride In tetrahydrofuran for 0.166667h; Inert atmosphere; Stage #3: Desyl chloride With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In tetrahydrofuran at 45℃; Inert atmosphere; optical yield given as %de; | 91% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride; water In dimethyl sulfoxide at 110℃; under 760.051 Torr; for 20h; Schlenk technique; Sealed tube; | 91% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; sodium nitrite In N,N-dimethyl-formamide at 90℃; for 4h; Schlenk technique; | 91% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium carbonate In ethanol; water for 24h; Heating; | 90% |
| With hydroxylamine hydrochloride; sodium hydroxide In ethanol; water for 1h; Reflux; | 44% |
| With hydroxylamine hydrochloride; sodium acetate In methanol; water at 20℃; | |
| With ammonium hydroxide hydrochloride; sodium acetate In methanol; water at 20℃; | |
| With hydroxylamine hydrochloride; sodium acetate In methanol at 120℃; |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dimethyl sulfoxide at 25℃; for 0.6h; regioselective reaction; | 90% |
-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
1228284-86-3
1-(2,4,6-trichlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In tetrachloromethane at 50 - 60℃; for 5h; | 90% |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
3041-83-6
(E)-4-chloro-4'-methylstilbene

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; | 89% |
-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
565176-99-0
(S)-1-(4′-chlorophenyl)-2-propanol

| Conditions | Yield |
|---|---|
| With lyophilized-rehydrated Debaryomyces hansenii cells; isopropyl alcohol In water at 28℃; for 1h; | 88% |
| With W110V mutated thermoanaerobacter pseudoethanolicus secondary alcohol dehydrogenase; nicotinamide adenine dinucleotide phosphate; isopropyl alcohol In aq. buffer at 50℃; pH=8; Reagent/catalyst; Enzymatic reaction; enantioselective reaction; | n/a |
-
-
188472-71-1
4-chloro-1-ethynyl-2-fluorobenzene

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In dimethyl sulfoxide at 120℃; for 12h; Schlenk technique; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; magnesium sulfate; N-ethyl-N,N-diisopropylamine In toluene at 60℃; for 6h; | 87% |
-
-
122-03-2
(4-isopropylbenzaldehyde)

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
1309065-22-2
(E)-1-chloro-4-(4-isopropylstyryl)benzene

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; | 87% |
-
-
17356-08-0
thiourea

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
90797-73-2
5‐(4‐chlorophenyl)‐4‐methyl‐2‐amino‐1,3‐thiazole

| Conditions | Yield |
|---|---|
| With cesium bicarbonate; Bromotrichloromethane In acetonitrile at 80℃; for 2h; | 87% |
-
-
134469-07-1
Benzimidazol-2-thiol

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride; dihydrogen peroxide; dimethyl sulfoxide In water at 135℃; for 0.5h; Sealed tube; Green chemistry; regioselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; potassium hydroxide In water at 100℃; for 24h; Inert atmosphere; regioselective reaction; | 87% |
-
-
100-52-7
benzaldehyde

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
1657-50-7
(E)-1-(4-chlorophenyl)-2-phenylethene

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; | 86% |
-
-
5720-07-0
4-methoxyphenylboronic acid

-
-
5586-88-9
1-(4-chlorophenyl)propan-2-one

-
-
121804-15-7
1-(4'-methoxy-[1,1'-biphenyl]-4-yl)propan-2-one

| Conditions | Yield |
|---|---|
| With (3-phenylallyl)(chloro)-[1,3-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazol-2-ylidene]palladium(II); potassium carbonate In ethanol; water at 80℃; for 4h; Suzuki-Miyaura Coupling; Sealed tube; Green chemistry; | 86% |
4-Chlorophenylacetone Specification
The 4-Chlorophenylacetone with CAS registry number of 5586-88-9 is also known as p-Chlorophenyl-2-propanone. The IUPAC name is 1-(4-Chlorophenyl)propan-2-one. It belongs to product categories of Aromatic Ketones (substituted). Its EINECS registry number is 226-986-4. In addition, the formula is C9H9ClO and the molecular weight is 168.62. This chemical is a clear light yellow liquid that stable at normal temperature and pressure and should be sealed in cool, dry place without light. During usint it, avoid contact with skin and eyes.
Physical properties about 4-Chlorophenylacetone are: (1)ACD/LogP: 2.03; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.03; (4)ACD/LogD (pH 7.4): 2.03; (5)ACD/BCF (pH 5.5): 20.7; (6)ACD/BCF (pH 7.4): 20.7; (7)ACD/KOC (pH 5.5): 304.47; (8)ACD/KOC (pH 7.4): 304.47; (9)#H bond acceptors: 1; (10)#Freely Rotating Bonds: 2; (11)Index of Refraction: 1.525; (12)Molar Refractivity: 45.35 cm3; (13)Molar Volume: 147.8 cm3; (14)Surface Tension: 36.9 dyne/cm; (15)Density: 1.14 g/cm3; (16)Flash Point: 116.3 °C; (17)Enthalpy of Vaporization: 47.33 kJ/mol; (18)Boiling Point: 236.5 °C at 760 mmHg; (19)Vapour Pressure: 0.0471 mmHg at 25 °C.
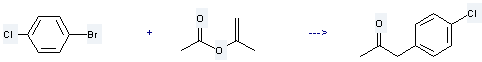
Preparation of 4-Chlorophenylacetone: it is prepared by reaction of 1-bromo-4-chloro-benzene with 2-acetoxy-propene. The reaction needs reagent tributyltin methoxide, catalyst dichlorobis(tri-o-tolylphosphine)palladium and solvent toluene at the temperature of 100 °C for 5 hours. The yield is about 73 %.
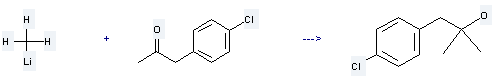
Uses of 4-Chlorophenylacetone: it is used to produce 1-(4-chloro-phenyl)-2-methyl-propan-2-ol by reaction with methyllithium. The reaction occurs with reagent diethyl ether and the yield is about 79 %.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CC(=O)CC1=CC=C(C=C1)Cl
2. InChI: InChI=1S/C9H9ClO/c1-7(11)6-8-2-4-9(10)5-3-8/h2-5H,6H2,1H3
3. InChIKey: WEJRYKSUUFKMBC-UHFFFAOYSA-N
Related Products
- 4-Chlorophenylacetone
- 55869-99-3
- 55870-50-3
- 55873-09-1
- 5587-42-8
- 55877-79-7
- 55878-47-2
- 5588-10-3
- 55881-03-3
- 55881-07-7
- 55881-52-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View