-
Name
Dichlorophenylphosphine
- EINECS 211-425-8
- CAS No. 644-97-3
- Article Data94
- CAS DataBase
- Density 1.319g/mLat 25°C(lit.)
- Solubility Slightly soluble in water, soluble in ethanol, acetone, diethyl ether
- Melting Point -51 °C(lit.)
- Formula C6H5Cl2P
- Boiling Point 224 °C at 760 mmHg
- Molecular Weight 178.985
- Flash Point 89.272 °C
- Transport Information UN 2845 4.2/PG 1
- Appearance Colorless to light yellow liquid
- Safety 26-28-36/37/39-43-45
- Risk Codes 17-25-34-29
-
Molecular Structure
-
Hazard Symbols
 F,
F, T
T
- Synonyms Phosphonousdichloride, phenyl- (6CI,8CI,9CI);NSC 66478;Phenylphosphorus dichloride;Phenyldichlorophosphine;Phenylphosphine dichloride;Phenylphosphinous dichloride;Phenylphosphonous acid dichloride;Phosphine, dichlorophenyl-;Benzenephosphorus Dichloride;
- PSA 13.59000
- LogP 3.10140
Synthetic route

| Conditions | Yield |
|---|---|
| With aluminium trichloride; phosphorus trichloride for 3h; Heating; | 96% |
| Stage #1: benzene With aluminum (III) chloride; phosphorus trichloride for 5h; Reflux; Sealed tube; Stage #2: With trichlorophosphate In Petroleum ether for 1h; Concentration; Reflux; Sealed tube; | 95.5% |
| With phosphorus trichloride for 2h; Time; Reflux; | 93.6% |

| Conditions | Yield |
|---|---|
| With phosphorus; phosphorus trichloride at 70 - 350℃; under 30003 Torr; for 6h; Concentration; Pressure; Temperature; | 94.35% |
| With phosphorus; phosphorus trichloride at 390℃; | 58.9% |
| With phosphorus; nickel; phosphorus trichloride at 390℃; Product distribution; other catalysts; | 33.1% |

| Conditions | Yield |
|---|---|
| With triphenylphosphine | 91% |

| Conditions | Yield |
|---|---|
| With phosphorus trichloride at 350℃; for 5h; | 85% |

-
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With aluminium trichloride; phosphorus trichloride for 5h; Heating; | 84% |

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In diethyl ether at 20℃; | 83% |
| With phosphorus pentachloride In toluene |

| Conditions | Yield |
|---|---|
| With tetraethylammonium iodide In benzene for 1h; | 79% |
| With phosphorous acid trimethyl ester; triethyl phosphite |
-
-
55045-25-5
phenyltrichlorophosphonium hexachlorophosphorate

-
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With diethyl phosphorylchloridite In benzene for 6h; also with EtOPCl2; | 72% |
| With difluoro diethylamino phosphine In benzene for 0.5h; | |
| With difluoro diethylamino phosphine In benzene for 0.5h; other substrates; |
-
-
23588-02-5
ethyl phenylphosphonochloridothioite

-
-
108-94-1
cyclohexanone

-
A
-
60340-72-9
chloroanhydride of phenyl-α-hydroxycyclohexylphosphinic acid

-
B
-
1779-48-2
phenylphosphinic acid

-
C
-
67176-71-0
C6H6ClOP

-
D
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With acetic acid at 0℃; for 240h; | A 50% B n/a C n/a D n/a |

-
-
75589-83-2
N,N-dimethylamino-P-phenyl-C-methylmethylenephosphine

-
-
931-59-9
benzenesulfenyl chloride

-
A
-
26990-26-1
S-phenyl-P-phenyldithiophosphonous chloride

-
B
-
38476-60-7
S,S-diphenyl-P-phenyldithiophosphonite

-
C
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| In acetonitrile | A n/a B 20% C n/a |


| Conditions | Yield |
|---|---|
| With aluminium trichloride; phosphorus trichloride at 140 - 150℃; for 5h; Product distribution; Mechanism; var. temps; var. molar proportion of reactants; var. time; | A 14% B 20% |

| Conditions | Yield |
|---|---|
| With phosphorus trichloride at 180℃; |
-
-
115-96-8
Tris(2-chloroethyl) phosphate

-
-
82123-74-8
phenylphosphonous acid dichloride; compound with aluminium trichloride (1:1)

-
A
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With phosphorus trichloride at 30℃; for 0.333333h; Product distribution; complexes of various arylphosphonous dichlorides with aluminum chloride; |

-
-
4895-65-2
phenyltetrachlorophosphorane

-
A
-
824-72-6
P,P-dichlorophenylphosphine oxide

-
B
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With Diethyl phosphate In benzene for 1h; Product distribution; (C2H5O)2PONa; |
-
-
4895-65-2
phenyltetrachlorophosphorane

-
A
-
3135-58-8
phenyl-β-chlorovinylphosphinic chloride

-
B
-
90876-11-2
phenyl-β-ethoxyvinylphosphinic chloride

-
C
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With phosphan; ethyl vinyl ether Yield given. Multistep reaction. Yields of byproduct given; |

-
-
13410-61-2
diphenyl phenylphosphonite

-
-
2171-93-9
phenyl-phosphonochloridous acid phenyl ester

-
A
-
101-02-0
triphenyl phosphite

-
B
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| at 140℃; Equilibrium constant; |

-
-
126215-38-1
C11H8ClF8OP

-
-
126215-34-7
Phosphorochloridous acid bis-(2,2,3,3,4,4,5,5-octafluoro-pentyl) ester

-
A
-
65611-17-8
tris(2,2,3,3,4,4,5,5-octafluoropentyl) phosphite

-
B
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| at 140℃; Equilibrium constant; |

-
-
55045-25-5
phenyltrichlorophosphonium hexachlorophosphorate

-
A
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With triethylsulfonium iodide |
-
-
4895-65-2
phenyltetrachlorophosphorane

-
-
109-92-2
ethyl vinyl ether

-
A
-
3135-58-8
phenyl-β-chlorovinylphosphinic chloride

-
B
-
90876-11-2
phenyl-β-ethoxyvinylphosphinic chloride

-
C
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With phosphan Yield given. Multistep reaction. Yields of byproduct given; |

-
-
149-74-6
dichloromethylphenylsilane

-
A
-
75-79-6
Methyltrichlorosilane

-
B
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With pyridine; aluminium trichloride; phosphorus trichloride 1) 80-90 deg C, 10 h, 2) reflux, 1 h; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
587-85-9
diphenylmercury(II)

-
-
7719-12-2, 52843-90-0
phosphorus trichloride

-
A
-
644-97-3
Dichlorophenylphosphine

-
B
-
100-56-1
phenylmercury(II) chloride

| Conditions | Yield |
|---|---|
| at 180℃; |

-
-
75-44-5
phosgene

-
-
638-21-1
phenylphosphane

-
A
-
7647-01-0
hydrogenchloride

-
B
-
644-97-3
Dichlorophenylphosphine

-
C
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
7446-70-0
aluminium trichloride

-
-
7719-12-2, 52843-90-0
phosphorus trichloride

-
-
71-43-2
benzene

-
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| bei Siedetemperatur; |


| Conditions | Yield |
|---|---|
| beim Erhitzen; |
-
-
60-29-7
diethyl ether

-
-
603-33-8
triphenylbismuthane

-
-
7719-12-2, 52843-90-0
phosphorus trichloride

-
A
-
5153-28-6
diphenylbismuth(III) chloride

-
B
-
644-97-3
Dichlorophenylphosphine

-
C
-
1079-66-9
chloro-diphenylphosphine

| Conditions | Yield |
|---|---|
| reagiert analog mit Arsentrichlorid; |

-
-
81861-09-8
dibromo-dichloro-phenyl-phosphorane

-
A
-
106-37-6
1.4-dibromobenzene

-
B
-
10035-10-6, 12258-64-9
hydrogen bromide

-
C
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| at 150℃; |

-
-
421-17-0
Trifluoromethylsulfenyl chloride

-
-
6002-55-7
1,3-Dikalium-1,2,3-triphenyltriphosphid

-
A
-
69646-22-6
CF3SP(Cl)C6H5

-
B
-
69646-19-1
(CF3S)2PC6H5

-
C
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at -50 to 20°C; | |
| In tetrahydrofuran |

-
-
421-17-0
Trifluoromethylsulfenyl chloride

-
-
3376-52-1
pentaphenylcyclopentaphosphane

-
A
-
69646-22-6
CF3SP(Cl)C6H5

-
B
-
69646-19-1
(CF3S)2PC6H5

-
C
-
644-97-3
Dichlorophenylphosphine


| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 0.5h; Arbuzov reaction; | 100% |
| In tetrahydrofuran at 0 - 20℃; for 20h; Inert atmosphere; Schlenk technique; | 95% |
| In tetrahydrofuran at 0℃; | 90% |

| Conditions | Yield |
|---|---|
| 100% |

| Conditions | Yield |
|---|---|
| Stage #1: (-)-menthol With n-butyllithium In tetrahydrofuran; hexane at 0℃; Inert atmosphere; Stage #2: Dichlorophenylphosphine In tetrahydrofuran; hexane at -78 - 20℃; for 15h; Inert atmosphere; | 100% |
| With R3N | |
| With pyridine |
-
-
780-24-5
dibenzylmercury(II)

-
-
644-97-3
Dichlorophenylphosphine

-
-
29949-68-6, 73365-16-9
benzyl-phenylchlorophosphine

| Conditions | Yield |
|---|---|
| at 60℃; for 24h; | 100% |
-
-
64065-06-1
LiCH2PMe2

-
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran -78 deg C; RT, 12 h; | 100% |
-
-
2398-37-0
3-methoxyphenyl bromide

-
-
644-97-3
Dichlorophenylphosphine

-
-
216164-54-4
bis(3-methoxyphenyl)(phenyl)phosphine oxide

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxyphenyl bromide With magnesium Metallation; Stage #2: Dichlorophenylphosphine Arylation; Stage #3: With water; dihydrogen peroxide In tetrahydrofuran Oxidation; Further stages.; | 100% |
| With magnesium Yield given; |
-
-
2963-66-8
2-(2'-Hydroxyphenyl)benzimidazole

-
-
644-97-3
Dichlorophenylphosphine

-
-
769124-06-3
3,4-benzimidazole-5,6-benzo-2-phenyl-1,3,2-oxazaphosphorinane

| Conditions | Yield |
|---|---|
| With triethylamine In toluene at 20℃; | 100% |
-
-
644-97-3
Dichlorophenylphosphine

-
-
590-17-0
cyanomethyl bromide

-
-
61806-56-2
bis(cyanomethyl)phenylphosphine

| Conditions | Yield |
|---|---|
| With iodine; zinc In tetrahydrofuran for 1h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| 100% |

-
-
644-97-3
Dichlorophenylphosphine

-
-
1017605-86-5
2-phenyl-4,5-[1,2(1,2-dicarba-closo-dodecaborano)]-1,3-diselena-2-phospha-cyclopentane

| Conditions | Yield |
|---|---|
| In diethyl ether C6H5PCl2 added at -78°C to Se-compound in ether; stirred overnight at room temp.; filtered off; concd.; crystallized from CH2Cl2 at room temp.; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: C46H60O10; Dichlorophenylphosphine With pyridine at 20 - 70℃; Stage #2: With dihydrogen peroxide In chloroform; water at 20℃; for 0.5h; | 100% |
-
-
1263143-03-8
3-bromo-2-(2-bromophenyl)benzo[b]thiophene

-
-
644-97-3
Dichlorophenylphosphine

-
-
1263143-04-9
10-phenyl-10H-benzo[b]phosphindolo[2,3-d]thiophene

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromo-2-(2-bromophenyl)benzo[b]thiophene With n-butyllithium In diethyl ether; hexane at -78 - 20℃; for 2h; Inert atmosphere; Stage #2: Dichlorophenylphosphine In diethyl ether; hexane at 20℃; for 3h; Inert atmosphere; | 100% |
| Stage #1: 3-bromo-2-(2-bromophenyl)benzo[b]thiophene With n-butyllithium; N,N,N,N,-tetramethylethylenediamine In diethyl ether at -78℃; for 1h; Inert atmosphere; Stage #2: Dichlorophenylphosphine In diethyl ether at -78 - 20℃; Inert atmosphere; | 70% |
| Stage #1: 3-bromo-2-(2-bromophenyl)benzo[b]thiophene With n-butyllithium In diethyl ether; hexane at -20℃; for 0.25h; Inert atmosphere; Stage #2: Dichlorophenylphosphine In diethyl ether; hexane at -20 - 20℃; for 15h; Inert atmosphere; | 68% |
-
-
644-97-3
Dichlorophenylphosphine

-
-
220405-40-3
2,2-difluoro-1,3-dimethyl-imidazolidine

-
-
1352439-95-2
C11H15F4N2P

| Conditions | Yield |
|---|---|
| In diethyl ether at -40℃; for 12h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 20℃; for 12h; | 100% |
-
-
1065189-00-5
(2-fluorophenyl)2PN(i-propyl)H

-
-
644-97-3
Dichlorophenylphosphine

-
-
1448244-21-0
C21H20ClF2NP2

| Conditions | Yield |
|---|---|
| Stage #1: (2-fluorophenyl)2PN(i-propyl)H With n-butyllithium In pentane at -78℃; for 1h; Inert atmosphere; Stage #2: Dichlorophenylphosphine In pentane at -70℃; for 1.5h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| at 95℃; for 1h; Inert atmosphere; | 100% |
| at 95℃; for 1h; Inert atmosphere; | 100% |
-
-
65094-35-1
2-((trimethylsilyl)methyl)quinoline

-
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 18h; Cooling; | 100% |
| In tetrahydrofuran at 0℃; |

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-4-methyl-phenol In chloroform at 65℃; for 24h; Inert atmosphere; Sealed tube; Large scale; | 99.1% |
| McCormack reaction; | 68% |
| With 2,6-di-tert-butyl-4-methyl-phenol In toluene at 59 - 86℃; for 24h; Temperature; Inert atmosphere; | 5.204 g |
-
-
644-97-3
Dichlorophenylphosphine

-
-
124-40-3
dimethyl amine

-
-
6143-71-1
bis(dimethylamino)phenyl phosphine

| Conditions | Yield |
|---|---|
| In diethyl ether at 0℃; for 2h; | 99% |
| In Petroleum ether for 0.5h; Ambient temperature; | 83% |
| In diethyl ether at 5℃; for 3h; | 82% |
-
-
644-97-3
Dichlorophenylphosphine

-
-
153260-18-5
[Sn(CH3)2(1,2-benzenedithiolate)]

| Conditions | Yield |
|---|---|
| In benzene for 0.25h; Ambient temperature; | 99% |
-
-
644-97-3
Dichlorophenylphosphine

-
-
157768-68-8
4,7-diisopropyl-2,2-dimethyl-1,3,2-benzodithiastannole

| Conditions | Yield |
|---|---|
| In benzene for 0.25h; Ambient temperature; | 99% |
-
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| In benzene for 0.25h; Ambient temperature; | 99% |
-
-
28170-13-0
thiobenzoic acid potassium salt

-
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1h; Substitution; | A 99% B n/a |


| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1h; Substitution; | A 99% B n/a |

-
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at -78 - 20℃; | 99% |
-
-
644-97-3
Dichlorophenylphosphine

-
-
122039-27-4
woollins’ reagent

| Conditions | Yield |
|---|---|
| With selenium; ammonia; sodium In toluene at -78℃; for 68h; Inert atmosphere; Schlenk technique; Reflux; | 99% |
| Stage #1: Dichlorophenylphosphine With sodium selenide In toluene for 64h; Heating; Stage #2: With selenium In toluene for 3.5h; Heating; | 82% |

| Conditions | Yield |
|---|---|
| With 1,8-bis(dimethylamino)naphthalene; HCl In 1,2-dimethoxyethane byproducts: (proton sponge)H(+)Cl(-); N2-atmosphere; addn. of proton sponge and then phosphine to borane, standing for 16 h; filtration, evapn. (vac.), extn. into Et2O, HCl addn., filtration, evapn., drying (vac.); elem. anal.; | 99% |

-
-
644-97-3
Dichlorophenylphosphine

-
-
1312761-78-6
[(η5-C5H5)(η5-C5H4CMe2(CH2)2C8H7PPh)ZrCl2]

| Conditions | Yield |
|---|---|
| In (2)H8-toluene Ar; ligand added dropwise to toluene-d8 soln. of Zr compd. (1:1 molar ratio), mixt. stirred for 30 min; NMR; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,8-dibromonaphthalene With n-butyllithium In diethyl ether at -80 - 20℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: Dichlorophenylphosphine In diethyl ether at -80 - 20℃; Inert atmosphere; Schlenk technique; Stage #3: With dihydrogen peroxide In dichloromethane for 2h; Inert atmosphere; Schlenk technique; | 99% |
-
-
1148-79-4
2,2':6,2''-terpyridine

-
-
2797-28-6
sodium tetrakis(pentafluorophenyl)-borate

-
-
644-97-3
Dichlorophenylphosphine

| Conditions | Yield |
|---|---|
| In dichloromethane Inert atmosphere; | 98.8% |

Dichlorophenylphosphine Consensus Reports
Dichlorophenylphosphine Standards and Recommendations
Dichlorophenylphosphine Specification
The Dichlorophenylphosphine, with the CAS registry number 644-97-3, is also known as Phenyldichlorophosphine. It belongs to the product categories of Chlorophosphines; Pharmaceutical Intermediates; Ligand; Organophosphine halide. Its EINECS number is 211-425-8. This chemical's molecular formula is C6H5Cl2P and molecular weight is 178.98. What's more, its systematic name is Phenylphosphonous dichloride. This colourless viscous liquid is commonly used in the synthesis of phosphine ligands. Dichlorophenylphosphine is commercially available. It may be prepared by a electrophilic substitution of benzene by phosphorus trichloride, catalyzed by aluminium chloride. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides, acids, heat and fire.
Physical properties of Dichlorophenylphosphine are: (1)ACD/LogP: 2.421; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.42; (4)ACD/LogD (pH 7.4): 2.42; (5)ACD/BCF (pH 5.5): 40.76; (6)ACD/BCF (pH 7.4): 40.76; (7)ACD/KOC (pH 5.5): 494.52; (8)ACD/KOC (pH 7.4): 494.52; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 13.59 Å2; (13)Flash Point: 89.272 °C; (14)Enthalpy of Vaporization: 44.176 kJ/mol; (15)Boiling Point: 224 °C at 760 mmHg; (16)Vapour Pressure: 0.1 mmHg at 25°C.
Preparation: this chemical can be prepared by benzene by heating. This reaction will need reagents PCl3, AlCl3 with the reaction time of 3 hours. The yield is about 96%.

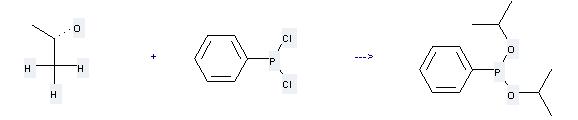
Uses of Dichlorophenylphosphine: it can be used to produce phenyl-phosphonous acid diisopropyl ester at the ambient temperature. It will need reagent pyridine and solvent hexane with the reaction time of 3 hours. The yield is about 76%.
When you are using this chemical, please be cautious about it as the following:
This chemical can cause buins, and is spontaneously flammable in air. When contact with water, it will liberate toxic gas. It is toxic if swallowed. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. After contact with skin, you must wash immediately with plenty of ... (to be specified by the manufacturer). When using it, you need wear suitable protective clothing, gloves and eye/face protection. In case of fire use ... (indicate in the space the precise type of fire-fighting equipment. If water increases the risk add - Never use water). In case of accident or if you feel unwell, you need to seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: ClP(Cl)c1ccccc1
(2)Std. InChI: InChI=1S/C6H5Cl2P/c7-9(8)6-4-2-1-3-5-6/h1-5H
(3)Std. InChIKey: IMDXZWRLUZPMDH-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 100mg/kg (100mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | National Technical Information Service. Vol. 0TS0555341, |
| mouse | LDLo | intraperitoneal | 100mg/kg (100mg/kg) | SKIN AND APPENDAGES (SKIN): HAIR: OTHER BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: TREMOR | National Technical Information Service. Vol. 0TS0555341, |
| rat | LD50 | oral | 200mg/kg (200mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. 0TS0555341, |
| rat | LDLo | intraperitoneal | 100mg/kg (100mg/kg) | SKIN AND APPENDAGES (SKIN): HAIR: OTHER LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | National Technical Information Service. Vol. 0TS0555341, |
Related Products
- Dichlorophenylphosphine
- 64497-95-6
- 644982-12-7
- 644982-20-7
- 644-98-4
- 64499-64-5
- 645-00-1
- 64502-13-2
- 64504-69-4
- 645-05-6
- 64506-46-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View