-
Name
Ethyl chloroacetate
- EINECS 203-294-0
- CAS No. 105-39-5
- Article Data114
- CAS DataBase
- Density 1.119 g/cm3
- Solubility 20 g/L (20 ºC)
- Melting Point -27 ºC
- Formula C4H7ClO2
- Boiling Point 147.6 ºC at 760 mmHg
- Molecular Weight 122.551
- Flash Point 65.6 ºC
- Transport Information UN 1181 6.1/PG 2
- Appearance colourless liquid
- Safety 45-61-7/9-16
- Risk Codes 23/24/25-50-10
-
Molecular Structure
-
Hazard Symbols
 T;
T;  N
N
- Synonyms Aceticacid, chloro-, ethyl ester (6CI,8CI,9CI);(Ethoxycarbonyl)methyl chloride;2-Chloroacetic acid ethyl ester;Chloroacetic acid ethyl ester;Ethyl 2-chloroacetate;Ethyl 2-monochloroacetate;Ethyl monochloracetate;Ethyl a-chloroacetate;NSC 8833;
- PSA 26.30000
- LogP 0.78830
Synthetic route

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B at 20℃; for 36h; Enzymatic reaction; | 97% |
| at 105℃; for 3h; Temperature; Molecular sieve; | 97.4% |
| F-4SK (H form) In tetrachloromethane at 80℃; for 5h; | 95% |

| Conditions | Yield |
|---|---|
| With thionyl chloride In ethanol; dichloromethane for 0.583333h; Cooling with ice; | 83% |
-
-
623-33-6
glycine ethyl ester hydrochloride

-
-
79-04-9
chloroacetyl chloride

-
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; toluene at 20℃; for 13h; Cooling with ice; | 80% |

| Conditions | Yield |
|---|---|
| With tertiary butyl chloride; [HB(3,5-(CF3)2Pz)3]Ag(THF) | 73% |
| With hydrogenchloride | |
| With hydrogenchloride; diethyl ether |

| Conditions | Yield |
|---|---|
| sulfuric acid In ethanol Heating; | 66% |

| Conditions | Yield |
|---|---|
| With potassium bromate; hydrogenchloride In N,N-dimethyl-formamide at 20℃; for 12h; | 63% |
-
-
623-73-4
diazoacetic acid ethyl ester

-
A
-
105-39-5
chloroacetic acid ethyl ester

-
B
-
1520-50-9
but-2-enedioic acid diethyl ester

| Conditions | Yield |
|---|---|
| With tertiary butyl chloride; bis(μ2-2,2,2',2'-tetramethyl-1,3-benzene-di(propanoato-O,O'))rhodium-bismuth at 39.84℃; for 2h; Inert atmosphere; | A 46% B n/a |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
140-29-4
phenylacetonitrile

-
A
-
74185-57-2
2-benzyl-5-ethoxy-1,3-oxazole

-
B
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| aluminium trichloride In neat (no solvent) at 25℃; | A 31% B n/a |

-
-
7064-36-0
4-chloro-5-methyl-isoxazole

-
-
64-17-5
ethanol

-
A
-
141-78-6
ethyl acetate

-
B
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With pyridine for 5h; Heating; | A n/a B 25% |

-
-
623-73-4
diazoacetic acid ethyl ester

-
-
507-20-0
tertiary butyl chloride

-
A
-
98551-46-3
2-chloro-3,3-dimethyl-butyric acid ethyl ester

-
B
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| Photolysis; | |
| Irradiation.mit UV-Licht; |

-
-
623-73-4
diazoacetic acid ethyl ester

-
A
-
26697-96-1
bis-(ethoxycarbonyl-chloro-chloromercurio-methyl)-mercury

-
B
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With mercury dichloride at 0℃; |
-
-
67-56-1
methanol

-
-
42345-82-4
(E)-1,2-dichloro-1-ethoxy-ethene

-
A
-
96-34-4
methyl chloroacetate

-
B
-
105-39-5
chloroacetic acid ethyl ester

-
-
56-23-5
tetrachloromethane

-
-
128-09-6
N-chloro-succinimide

-
-
2678-54-8
ketene diethyl acetal

-
A
-
74-85-1
ethene

-
B
-
105-39-5
chloroacetic acid ethyl ester

-
-
60-29-7
diethyl ether

-
-
191340-22-4
chloro-acetyl iodide

-
A
-
75-03-6
ethyl iodide

-
B
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 25℃; | |
| at 25℃; bei mehrtaegiger Einw. unter Lichtausschuss; reagiert analog mit Methylbutylaether; |


| Conditions | Yield |
|---|---|
| at 25℃; unter Ausschluss von Licht; |
-
-
64-17-5
ethanol

-
-
80944-05-4
1-ethoxy-2,2-dichloro-ethanol

-
-
151-50-8
potassium cyanide

-
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 20℃; |


| Conditions | Yield |
|---|---|
| zweckmaessig in Gegenwart von Katalysatoren; |
-
-
64-17-5
ethanol

-
-
17368-73-9
N-acetyl-2-chloroacetamide

-
A
-
141-78-6
ethyl acetate

-
B
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| Product distribution; im Verhaeltnis 99:1; |

-
-
64-17-5
ethanol

-
-
15322-68-6
chloroacetyl-propionyl-amine

-
A
-
105-37-3
Ethyl propionate

-
B
-
105-39-5
chloroacetic acid ethyl ester

-
-
64-17-5
ethanol

-
-
4960-82-1
dichlorodiacetamide

-
A
-
105-39-5
chloroacetic acid ethyl ester

-
B
-
79-07-2
Chloroacetamide

-
-
64-17-5
ethanol

-
-
7218-27-1
N-(2-chloroethanoyl)benzamide

-
A
-
93-89-0
benzoic acid ethyl ester

-
B
-
105-39-5
chloroacetic acid ethyl ester

-
-
64-17-5
ethanol

-
-
79628-75-4
3-(2-amino-4-methyl-anilino)-2-chloro-crotonic acid ethyl ester

-
A
-
1792-41-2
2,5-dimethylbenzimidazole

-
B
-
105-39-5
chloroacetic acid ethyl ester

-
-
42345-82-4
(E)-1,2-dichloro-1-ethoxy-ethene

-
-
123-51-3
i-Amyl alcohol

-
A
-
5326-92-1
3-methylbutyl chloroacetate

-
B
-
105-39-5
chloroacetic acid ethyl ester

-
-
42345-82-4
(E)-1,2-dichloro-1-ethoxy-ethene

-
-
111-70-6
n-heptan1ol

-
A
-
34589-22-5
heptyl 2-chloroacetate

-
B
-
105-39-5
chloroacetic acid ethyl ester

-
-
80944-05-4
1-ethoxy-2,2-dichloro-ethanol

-
-
151-50-8
potassium cyanide

-
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With ethanol at 20℃; |
-
-
42345-82-4
(E)-1,2-dichloro-1-ethoxy-ethene

-
-
110-15-6
succinic acid

-
A
-
108-30-5
succinic acid anhydride

-
B
-
105-39-5
chloroacetic acid ethyl ester

-
-
42345-82-4
(E)-1,2-dichloro-1-ethoxy-ethene

-
-
89-78-1
menthol

-
A
-
106916-72-7
2-isopropyl-5-methylcyclohexyl chloroethanoate

-
B
-
105-39-5
chloroacetic acid ethyl ester

-
-
42345-82-4
(E)-1,2-dichloro-1-ethoxy-ethene

-
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With alcohols | |
| With mono-basic organic acids | |
| With oxalic acid |
-
-
95-77-2
3,4-dichlorophenol

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
62855-72-5
ethyl 2-(3,4-dichlorophenoxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 80℃; for 18h; | 100% |
| With ethanol; sodium ethanolate |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
109-94-4
formic acid ethyl ester

-
-
33142-21-1
ethyl 2-chloro-3-oxopropanoate

| Conditions | Yield |
|---|---|
| With sodium ethanolate In tert-butyl methyl ether at 0 - 20℃; Inert atmosphere; Large scale; | 100% |
| With sodium ethanolate In tert-butyl methyl ether at 0 - 20℃; Inert atmosphere; Large scale; | 100% |
| With potassium tert-butylate In tetrahydrofuran at 0 - 20℃; for 16h; Inert atmosphere; | 95% |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
89124-45-8
acetic acid ethylester sulfonic acid

| Conditions | Yield |
|---|---|
| With sodium sulfate In ethanol; water | 100% |
| With potassium sulfite; ethanol |
-
-
1666-13-3
diphenyl diselenide

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
51364-94-4
ethyl 2-(phenylselanyl)acetate

| Conditions | Yield |
|---|---|
| Stage #1: diphenyl diselenide With sodium tetrahydroborate In ethanol at 0℃; for 0.166667h; Inert atmosphere; Stage #2: chloroacetic acid ethyl ester In ethanol for 1h; Inert atmosphere; | 100% |
| Stage #1: diphenyl diselenide With sodium tetrahydroborate; ethanol at 0 - 25℃; for 1h; Stage #2: chloroacetic acid ethyl ester at 25℃; for 2h; | 98% |
| Stage #1: diphenyl diselenide With sodium tetrahydroborate In ethanol for 0.166667h; Stage #2: chloroacetic acid ethyl ester In ethanol at 50 - 55℃; for 0.75h; | 94% |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
85666-09-7
E-3-m-hydroxystyrylpyridine

-
-
79131-36-5
1-Ethoxycarbonylmethyl-4-[(E)-2-(3-hydroxy-phenyl)-vinyl]-pyridinium; chloride

| Conditions | Yield |
|---|---|
| In ethanol for 10h; Heating; | 100% |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
76799-30-9
1-<2,6-bis(benzyloxy)-4-hydroxyphenyl>-3-<3-(benzyloxy)-4-methoxyphenyl>propane

-
-
76820-12-7
1-<2,6-bis(benzyloxy)-4-(carbethoxymethoxy)phenyl>-3-(3-hydroxy-4-methoxyphenyl)propane

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; for 22h; | 100% |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
63240-52-8
2,3',6-tris(benzyloxy)-4-hydroxy-4'-methoxydihydrochalcone

-
-
76799-27-4
2,3',6-tris(benzyloxy)-4-(carbethoxymethoxy)-4'-methoxydihydrochalcone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 40 - 65℃; for 19h; | 100% |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
588679-10-1
ethyl 2-(4-bromo-2-chlorophenoxy)acetate

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromo-2-chlorophenol With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 1h; Stage #2: chloroacetic acid ethyl ester In N,N-dimethyl-formamide at 20℃; | 100% |
-
-
29799-07-3
4-adamantylphenol

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
52804-25-8
(4-adamantyl-1-yl-phenoxy) acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 94.6% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 12h; Inert atmosphere; | 94.6% |
-
-
372-20-3
3-fluorophenol

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
777-70-8
(3-fluoro-phenoxy)-acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Heating / reflux; | 100% |
| With potassium carbonate In acetone for 8h; Reflux; | 70% |
| With potassium carbonate In acetonitrile for 2h; Reflux; |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
119-36-8
methyl salicylate

-
-
22511-42-8
methyl 2-(2-ethoxy-2-oxoethoxy)benzoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 30 - 65℃; for 18h; | 100% |
| With potassium carbonate In acetone for 11h; Reflux; | 94% |
| With potassium iodide; potassium carbonate In acetonitrile | |
| With potassium carbonate In N,N-dimethyl-formamide at 65 - 75℃; |
-
-
394-32-1
1-(5-fluoro-2-hydroxyphenyl)ethan-1-one

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
34849-57-5
(2-acetyl-4-fluorophenoxy)acetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 16h; Reflux; Inert atmosphere; | 100% |
-
-
3964-56-5
4-bromo-2-chlorophenol

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
588679-10-1
ethyl 2-(4-bromo-2-chlorophenoxy)acetate

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromo-2-chlorophenol; chloroacetic acid ethyl ester With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 1h; Inert atmosphere; Stage #2: chloroacetic acid ethyl ester In N,N-dimethyl-formamide at 20℃; | 100% |
-
-
21392-48-3
7-hydroxy-8-methyl-4-phenyl-chromen-2-one

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
307547-31-5
ethyl (8-methyl-4-phenyl-2H-1-benzopyran-2-on-7-yloxy)-acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Reflux; | 100% |
| With potassium carbonate In acetone for 6h; Reflux; | 96.94% |
-
-
1245623-59-9
5-(5-chloro-2-thienyl)-4-(2-methoxyethyl)-2,4-dihydro-3H-1,2,4-triazol-3-one

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
1245623-60-2
ethyl [3-(5-chloro-2-thienyl)-4-(2-methoxyethyl)-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl]acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; for 6.5h; | 100% |
| With potassium carbonate In acetonitrile at 80℃; for 6.5h; | 100% |
| With potassium carbonate In acetonitrile at 80℃; for 6.5h; | 100% |
-
-
7480-35-5, 13286-59-4, 74165-73-4, 126456-43-7, 136030-00-7, 140632-19-5, 140632-20-8
(1S,2R)-1-amino-2-indanol

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
862095-79-2
indeno[2,1-b]-1,4-oxazin-3(2H)-one,4,4a,9,9a-tetrahydro-,(4aR,9aS)-

| Conditions | Yield |
|---|---|
| Stage #1: (1S,2R)-1-amino-2-indanol With sodium hydride In tetrahydrofuran at 0 - 70℃; for 0.666667h; Stage #2: chloroacetic acid ethyl ester In tetrahydrofuran for 2.5h; Heating / reflux; | 100% |
| With sodium hydride In tetrahydrofuran | 85% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In acetonitrile for 18h; Reflux; | 100% |
| Stage #1: bis[(2-pyridyl)methyl]amine With sodium hydrogencarbonate In acetonitrile for 0.166667h; Stage #2: chloroacetic acid ethyl ester In acetonitrile for 5h; Reflux; Inert atmosphere; | |
| With triethylamine In ethanol for 12h; Reflux; |
-
-
105-39-5
chloroacetic acid ethyl ester

-
-
38663-85-3
2-methoxyethyl isothiocyanate

-
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| Stage #1: 2-methoxyethyl isothiocyanate; 4-aminoethylbenzene In ethanol at 78℃; for 12h; Knoevenagel Condensation; Stage #2: chloroacetic acid ethyl ester With sodium acetate In ethanol at 78℃; for 5h; regioselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 16h; Reflux; Inert atmosphere; | 100% |
-
-
28668-95-3
3-mercapto-5H-1,2,4-triazino[5,6-b]indole

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
189325-51-7
ethyl ester of (1,2,4-triazino[5,6-b]indolyl-3-thio)acetic acid

| Conditions | Yield |
|---|---|
| With sodium In methanol; N,N-dimethyl-formamide at 60 - 100℃; for 15h; Alkylation; | 99.5% |
| Stage #1: 3-mercapto-5H-1,2,4-triazino[5,6-b]indole With potassium carbonate microwave irradiation; Stage #2: chloroacetic acid ethyl ester for 0.0416667h; microwave irradiation; | 89% |
-
-
106-41-2
4-bromo-phenol

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
6964-29-0
ethyl 2-(4-bromophenoxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; | 99.3% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 4h; | 99% |
| With potassium carbonate In acetone Reflux; | 87% |

| Conditions | Yield |
|---|---|
| at -5 - 20℃; for 2h; Inert atmosphere; | 99.3% |

-
-
540-38-5
4-Iodophenol

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
90794-33-5
4-iodophenoxyacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; Inert atmosphere; Schlenk technique; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide for 16h; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 96% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; |
-
-
52542-59-3, 192520-17-5
bis(triphenylphosphineiminium) pentacarbonylmanganate(1-)

-
-
105-39-5
chloroacetic acid ethyl ester

-
A
-
10170-69-1
dimanganese decacarbonyl

-
B
-
14100-30-2
pentacarbonylchloromanganese(I)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran (N2 or Ar); 25°C, rapid mixing; determination by NMR; | A 1% B 0% C 99% |
| In chloroform-d1 (N2 or Ar); 25°C, rapid mixing; determination by NMR; | A 1% B 0% C 99% |

-
-
66129-30-4
6-phenyl-[1,2,4]triazolo[4,3-b]pyridazine-3(2H)-thione

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
1033038-23-1
ethyl-2-(6-phenyl-[1,2,4]triazolo[4,3-b]pyridazin-3-ylsulfanyl)acetate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 20℃; for 3h; | 99% |

| Conditions | Yield |
|---|---|
| With sodium iodide In acetone at 20℃; for 18h; | 99% |

| Conditions | Yield |
|---|---|
| With sodium In ethanol at 20℃; for 4h; | 99% |
-
-
40064-34-4
piperidine-4,4-diol hydrochloride

-
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 75℃; | 99% |
-
-
103027-41-4
1-(tert-butyldimethylsilyl)-4-(4',4',5',5'-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indol-5-ol

-
-
105-39-5
chloroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 115℃; for 2h; | 99% |
-
-
5150-42-5
2,3-dimethoxyphenol

-
-
105-39-5
chloroacetic acid ethyl ester

-
-
861085-96-3
ethyl (2,3-dimethoxyphenoxy)acetate

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dimethoxyphenol With sodium hydride In tetrahydrofuran; dimethyl sulfoxide at 0℃; for 0.5h; Inert atmosphere; Stage #2: chloroacetic acid ethyl ester In tetrahydrofuran; dimethyl sulfoxide at 20℃; for 15h; Inert atmosphere; | 98.8% |
Ethyl chloracetate Consensus Reports
Reported in EPA TSCA Inventory.
Ethyl chloracetate Standards and Recommendations
DOT Classification: 6.1; Label: Poison
Ethyl chloracetate Specification
The CAS registry number of Ethyl chloracetate is 105-39-5. Its EINECS registry number is 203-294-0. The IUPAC name is ethyl 2-chloroacetate. In addition, the molecular formula is C4H7ClO2. What's more, it is a kind of colourless liquid and can be used as a pharmaceutical intermediate. It is incompatible with acids, bases, oxidizing agents and reducing agents.
Physical properties about this chemical are: (1)ACD/LogP: 0.94; (2)ACD/LogD (pH 5.5): 0.94; (3)ACD/LogD (pH 7.4): 0.94; (4)ACD/BCF (pH 5.5): 3.05; (5)ACD/BCF (pH 7.4): 3.05; (6)ACD/KOC (pH 5.5): 77.3; (7)ACD/KOC (pH 7.4): 77.3; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 3; (10)Polar Surface Area: 26.3 Å2; (11)Index of Refraction: 1.411; (12)Molar Refractivity: 27.2 cm3; (13)Molar Volume: 109.4 cm3; (14)Polarizability: 10.78 ×10-24cm3; (15)Surface Tension: 29.2 dyne/cm; (16)Density: 1.119 g/cm3; (17)Flash Point: 65.6 °C; (18)Enthalpy of Vaporization: 40.43 kJ/mol; (19)Boiling Point: 147.6 °C at 760 mmHg; (20)Vapour Pressure: 4.39 mmHg at 25°C.
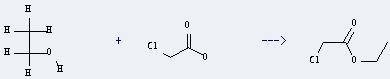
Preparation of Ethyl chloracetate: it can be prepared by chloroacetic acid and ethanol. This reaction will need reagents P2O5, CuSO4 and Na2SO4. The reaction time is 2 hours by heating. The yield is about 45%.
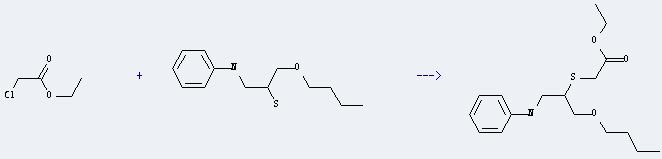
Uses of Ethyl chloracetate: it can react with 1-(N-phenylamino)-3-butoxypropane-2-thiol to get (1-butoxymethyl-2-phenylamino-ethylsulfanyl)-acetic acid ethyl ester. This reaction will need reagent NaOH and solvent H2O. The reaction time is 1 hours at reaction temperature of 30-40 °C. The yield is about 32%.
When you are using this chemical, please be cautious about it as the following:
This chemical is flammable. And it is toxic by inhalation, in contact with skin and if swallowed. Moreover, it is very toxic to aquatic organisms. During using it, you should keep away from sources of ignition. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.). In addition, you should avoid release to the environment and you can refer to special instructions/safety data sheets. Besides, keep container tightly closed and in a well-ventilated place after using it.
You can still convert the following datas into molecular structure:
(1)SMILES: ClCC(=O)OCC
(2)InChI: InChI=1/C4H7ClO2/c1-2-7-4(6)3-5/h2-3H2,1H3
(3)InChIKey: VEUUMBGHMNQHGO-UHFFFAOYAS
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 350mg/kg (350mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 30(5), Pg. 59, 1986. | |
| mouse | LD50 | subcutaneous | 250mg/kg (250mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | Japanese Journal of Pharmacology. Vol. 3, Pg. 99, 1954. |
| rabbit | LD50 | skin | 230mg/kg (230mg/kg) | Toxicology and Applied Pharmacology. Vol. 42, Pg. 417, 1977. | |
| rat | LC50 | inhalation | 3330uL/m3/4H (3.33mL/m3) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: TREMOR LUNGS, THORAX, OR RESPIRATION: DYSPNEA | BG Chemie: Toxicological Evaluations, Five Potential Health Hazards of Existing Chemicals. Vol. 5, Pg. 71, 1993. |
| rat | LD50 | oral | 180mg/kg (180mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA SKIN AND APPENDAGES (SKIN): HAIR: OTHER | BG Chemie: Toxicological Evaluations, Five Potential Health Hazards of Existing Chemicals. Vol. 5, Pg. 71, 1993. |
| rat | LD50 | skin | 161mg/kg (161mg/kg) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" SKIN AND APPENDAGES (SKIN): HAIR: OTHER | BG Chemie: Toxicological Evaluations, Five Potential Health Hazards of Existing Chemicals. Vol. 5, Pg. 71, 1993. |
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 105401-43-2
- 10540-29-1
- 10540-31-5
- 10540-39-3
- 105404-89-5
- 105404-97-5
- 105405-00-3
- 105405-04-7
- 105405-51-4
- 10541-56-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View