-
Name
L-(-)-Malic Acid
- EINECS 202-601-5
- CAS No. 97-67-6
- Article Data127
- CAS DataBase
- Density 1.641 g/cm3
- Solubility soluble in water
- Melting Point 101-103 °C(lit.)
- Formula C4H6O5
- Boiling Point 306.4 °C at 760 mmHg
- Molecular Weight 134.089
- Flash Point 153.4 °C
- Transport Information
- Appearance clear colourless solution
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms (2S)-2-Hydroxybutanedioic acid;(-)-Hydroxysuccinic acid;(-)-Malic acid;Apple acid;Hydroxysuccinnic acid (-);Butanedioic acid, hydroxy-, (2S)-;(-)-L-Malic acid;S-2-Hydroxybutanedioic acid;L-Malate;Malic acid, L-;Butanedioic acid, hydroxy-, (S)- (9CI);L-Apple acid;L(-)-Malic acid;Malic Acid;L-Maleic Acid;Hydroxybutanedioic acid, (S)-;84781-39-5;(S)-Malic acid;(-)-1-Hydroxy-1,2-ethanedicarboxylic acid;S-(-)-Malic acid;L-(-)-Malic acid;L-2-Hydroxybutanedioic acid;2-Hydroxybutanedioic acid, (S)-;
- PSA 94.83000
- LogP -1.09340
Synthetic route
-
-
80513-23-1
(R)-4,4,4-trichloro-3-hydroxybutanoic acid

-
-
97-67-6
(S)-Malic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide Ambient temperature; | 90% |
-
-
59025-03-5
(S)-acetoxysuccinic anhydride

-
-
97-67-6
(S)-Malic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide Ambient temperature; | 76% |
-
-
514829-23-3
(2S,4S)-2-(tert-butyl)-5-oxo-1,3-dioxolane-4-acetic acid isopropyl ester

-
A
-
97-67-6
(S)-Malic acid

-
B
-
514829-25-5
(2S)-2-hydroxybutanedioic acid 4-isopropyl ester

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran Heating; | A n/a B 66% |

| Conditions | Yield |
|---|---|
| With formaldehyd; TETRACYCLINE; paraquat dichloride In water at 37℃; for 4h; Clostridium formicoaceticum,phosphate buffer; | 52% |
| With phosphate buffer; hydrogen at 170℃; Rate constant; Thermodynamic data; enzymatic hydrolysis by fumarase, ΔS, ΔH, ΔG; also with absence of buffer; | |
| With fumarase |
-
-
16493-64-4
(S)-4,4,4-trichloro-3-hydroxybutanoic acid

-
A
-
97-67-6
(S)-Malic acid

-
B
-
110-17-8
(2E)-but-2-enedioic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 24h; | A 1.14 g B 5% |
-
-
71-23-8
propan-1-ol

-
-
56-84-8
L-Aspartic acid

-
A
-
97-67-6
(S)-Malic acid

-
B
-
110-17-8
(2E)-but-2-enedioic acid

| Conditions | Yield |
|---|---|
| bei der Einw. von Bact.coli; |


| Conditions | Yield |
|---|---|
| With fructose 1,6-bisphosphate trisodium; nicotinamide adenine dinucleotide red. form; R171W mutant In water at 25℃; Rate constant; other mutant enzyme; | |
| With kidney enzyme | |
| With homogenate from rat-tumors |

| Conditions | Yield |
|---|---|
| With penicillium-artene | |
| With Aspergillus niger |

| Conditions | Yield |
|---|---|
| bei der Einw. von Bac. pyocyaneus; |

| Conditions | Yield |
|---|---|
| Bei der Einw. des Yoghurtferments; | |
| durch das Yoghurtferment; | |
| durch Hefe; | |
| With cis-nitrous acid | |
| With hydrogenchloride; sodium nitrite In water for 24h; Ambient temperature; | 100 % Spectr. |

| Conditions | Yield |
|---|---|
| With hydrogenchloride Irradiation.bei der UV-Licht; | |
| With hydrogenchloride Irradiation.bei der UV-Licht;Kinetik und Quantenausbeute der Reaktion; | |
| With sulfuric acid; sodium nitrite Irradiation.bei der UV-Licht; |

| Conditions | Yield |
|---|---|
| With propan-1-ol bei der Einw. von Bact. coli; | |
| With propan-1-ol bei der Einw. von ruhenden Bact. coli; |

| Conditions | Yield |
|---|---|
| With ammonium molybdate; ammonia man erhaelt die freie Saeure durch Ausfaellung des Molybdaens mit Schwefelwasserstoff in salpetersaurer Loesung, Umsetzung mit Bleinitrat und Zerlegen des Bleisalzes mit Schwefelwasserstoff; | |
| With methanol; Cinchonin | |
| With acetone; Cinchonin | |
| With Cinchonin | |
| With D-tartaric acid |

| Conditions | Yield |
|---|---|
| With alkali Hydrolysis.bei der Hydrolyse der Dinatriumsalze; | |
| With water Hydrolysis.bei der Hydrolyse der Dinatriumsalze; |
-
-
16045-92-4
chlorosuccinic acid

-
-
97-67-6
(S)-Malic acid

| Conditions | Yield |
|---|---|
| With ammonium hydroxide | |
| With sodium hydroxide | |
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With alkali Hydrolysis.bei der Hydrolyse der Kupfer(II)-salze; | |
| With water Hydrolysis.bei der Hydrolyse der Kupfer(II)-salze; | |
| Hydrolysis.in saurer oder anfangs neutraler Loesung; |
-
-
923-06-8, 584-98-5
bromosuccinic acid

-
-
97-67-6
(S)-Malic acid

| Conditions | Yield |
|---|---|
| With water | |
| With silver(l) oxide | |
| With mercury(II) oxide | |
| With TlOH | |
| With palladium dihydroxide |
-
-
50-99-7, 59-23-4, 921-60-8, 1949-88-8, 1990-29-0, 2152-76-3, 2595-97-3, 2595-98-4, 3458-28-4, 4205-23-6, 5934-56-5, 5978-95-0, 5987-68-8, 6027-89-0, 6038-51-3, 7635-11-2, 10030-80-5, 15572-79-9, 19163-87-2, 23567-25-1, 26566-61-0, 30077-17-9, 31103-86-3, 39665-52-6, 40866-07-7, 58367-01-4, 58407-05-9, 58407-06-0, 83198-69-0, 83198-70-3, 83198-71-4, 93780-23-5, 145920-48-5
glucose

-
-
97-67-6
(S)-Malic acid

| Conditions | Yield |
|---|---|
| With aspergillus-artene | |
| With Rhizopus nigricans |

| Conditions | Yield |
|---|---|
| With enzyme compound from muscles; oxygen | |
| With methylene blue bei der Einw. von Enzymen aus Muskelgewebe von Warmbluetern ('Fumarase'); | |
| With enzyme compound from muscles; methylene blue | |
| With oxygen bei der Einw. von Enzymen aus Muskelgewebe von Warmbluetern ('Fumarase'); |

| Conditions | Yield |
|---|---|
| With methylene blue in Gegenwart von Muskelbrei oder Muskelextrakt; | |
| With oxygen in Gegenwart von Muskelbrei oder Muskelextrakt; | |
| With oxygen in Gegenwart von Muskelbrei oder Muskelextrakt; | |
| With methylene blue in Gegenwart von Muskelbrei oder Muskelextrakt; |

| Conditions | Yield |
|---|---|
| With bacterium succinicum |

| Conditions | Yield |
|---|---|
| With malic enzymes; reduced triphosphopyridinenucleotide | |
| With malic enzymes; reduced triphosphopyridinenucleotide |

| Conditions | Yield |
|---|---|
| With propan-1-ol; ammonium chloride bei der Einw. von ruhenden Bact. coli; |

| Conditions | Yield |
|---|---|
| With acetyl-coenzyme-A | |
| With yeast-juice from maceration | |
| With acetyl-coenzyme-A |
-
-
300-85-6, 625-71-8
3-Hydroxybutyric acid

-
-
97-67-6
(S)-Malic acid

| Conditions | Yield |
|---|---|
| bei der Einw. von Enzymen aus Rinderleberextrakt; | |
| With oxygen bei der Einw. von Enzymen aus Rinderleberextrakt; |

| Conditions | Yield |
|---|---|
| bei der Einw. von Aspergillus fumaricus, der jahrelang auf Kartoffeln gezuechtet wurde; | |
| With aspergillus-artene |

| Conditions | Yield |
|---|---|
| With Sucrose |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; calcium hypochlorite In water at 60℃; for 2h; Product distribution; various substrates, method for the structure analysis; |
-
-
17013-01-3
fumaric acid disodium salt

-
-
97-67-6
(S)-Malic acid

| Conditions | Yield |
|---|---|
| With Genus Brevibacterium In water at 37℃; Yield given; |

| Conditions | Yield |
|---|---|
| With thionyl chloride | 100% |
| With thionyl chloride at 0 - 20℃; Inert atmosphere; | 100% |
| With cationite KY-2 resin | 99% |
-
-
97-67-6
(S)-Malic acid

-
-
42890-76-6
(S)-1,2,4-butanetriol

| Conditions | Yield |
|---|---|
| With Trimethyl borate; dimethylsulfide borane complex In tetrahydrofuran at -5℃; for 51h; | 100% |
| With dimethylsulfide borane complex In tetrahydrofuran for 1h; | 100% |
| With Trimethyl borate; dimethylsulfide borane complex In tetrahydrofuran; methanol | 100% |

| Conditions | Yield |
|---|---|
| for 8h; Inert atmosphere; Reflux; | 100% |
-
-
97-67-6
(S)-Malic acid

-
-
108-94-1
cyclohexanone

-
-
153011-57-5
(5S)-(2,2-Cyclohexylidene-4-oxo-1,3-dioxolan-5-yl)acetic acid

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In diethyl ether at 0℃; for 1h; | 100% |
| With pyridinium p-toluenesulfonate In toluene for 16h; Reflux; | 92% |
| With boron trifluoride diethyl etherate In diethyl ether at 0 - 20℃; | 81% |

| Conditions | Yield |
|---|---|
| for 2h; Heating; | 100% |
| for 3h; Heating; | 99% |
| for 18h; Inert atmosphere; Reflux; | 98% |
-
-
97-67-6
(S)-Malic acid

-
-
407-25-0
trifluoroacetic anhydride

-
-
99529-36-9
(3S)-2,5-dioxotetrahydrofuran-3-yl trifluoroacetate

| Conditions | Yield |
|---|---|
| at 0 - 20℃; for 3h; | 100% |
| In 1,4-dioxane at 75℃; for 2h; | 97% |
| for 2h; Ambient temperature; |

| Conditions | Yield |
|---|---|
| With bis-(2-oxo-3-oxazolidinyl)phosphoryl chloride; triethylamine | 100% |
| With N,N-bis[2-oxo-3-oxazolidinyl]phosphorodiamidic chloride; triethylamine In acetonitrile Esterification; |

| Conditions | Yield |
|---|---|
| In methanol; ethanol at 20℃; for 2h; Darkness; | 100% |
| In ethanol for 2 - 4h; Product distribution / selectivity; Reflux; Industry scale; | 93.6% |
| In ethanol for 2 - 4h; Product distribution / selectivity; Reflux; | 93.6% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 0.5h; | 100% |
| In tetrahydrofuran Product distribution / selectivity; |
-
-
97-67-6
(S)-Malic acid

-
-
1174122-63-4
(R)-7-[3-amino-4-(2,4,5-trifluorophenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo [1,5-a]pyrazine-1-carboxylic acid

-
-
1242332-77-9
(R)-7-[3-amino-4-(2,4,5-trifluorophenyl)-butyryl]-3-trifluoromethyl-5,6,7,8-tetrahydro-imidazo [1,5-a]pyrazine-1-carboxylic acid (2S)-malic acid salt

| Conditions | Yield |
|---|---|
| In methanol; water for 0.5h; | 100% |
| In methanol; water for 0.5h; | 98.92% |
-
-
97-67-6
(S)-Malic acid

-
-
1269662-73-8
(E)-N-[4-[[3-chloro-4-(pyridin-2-ylmethoxy)phenyl]amino]-3-cyano-7-ethoxy-6-quinolinyl]-3-[(2R)-1-methylpyrrolidin-2-yl]propan-2-enoylamine

-
-
1397922-62-1
(E)-N-[4-[[3-chloro-4-(2-pyridylmethoxy)phenyl]amino]-3-cyano-7-ethoxy-6-quinolyl]-3-[(2R)-1-methylpyrrolidin-2-yl]prop-2-enamide L-malate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20 - 30℃; for 4h; Inert atmosphere; | 100% |
| In dichloromethane at 20 - 30℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| In methanol; water at 20℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 3h; | 100% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 3h; | 100% |
-
-
97-67-6
(S)-Malic acid

-
-
52079-23-9
(3S)-3-hydroxydihydrofuran-2(3H)-one

| Conditions | Yield |
|---|---|
| Stage #1: (S)-Malic acid With trifluoroacetic anhydride at 25℃; for 2h; Stage #2: With methanol for 12h; Stage #3: With borane-THF more than 3 stages; | 99.9% |
| Stage #1: (S)-Malic acid With toluene-4-sulfonic acid; 2,2-dimethoxy-propane Stage #2: With borane-THF |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 35℃; | 99% |
| With thionyl chloride at 0 - 35℃; | 99% |
| With hydrogenchloride In water for 4h; Reflux; | 96% |
-
-
302-17-0
chloral hydrate

-
-
97-67-6
(S)-Malic acid

-
-
196512-77-3
(2RS,5S)-5-carboxymethyl-2-trichloromethyl-4-oxo-1,3-dioxolane

| Conditions | Yield |
|---|---|
| With sulfuric acid for 10h; Ambient temperature; | 99% |
| With sulfuric acid at 20℃; for 3h; | 93% |
| With sulfuric acid at 20℃; for 3h; Inert atmosphere; | 93% |
| In sulfuric acid at 0 - 20℃; | 52% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 0.0833333h; | 99% |

| Conditions | Yield |
|---|---|
| In water at 95℃; | 99% |

-
-
97-67-6
(S)-Malic acid


| Conditions | Yield |
|---|---|
| In methanol; water for 0.5h; | 98.92% |
-
-
97-67-6
(S)-Malic acid


| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 0.5h; Reflux; | 98.5% |

| Conditions | Yield |
|---|---|
| In tert-butyl methyl ether; water; isopropyl alcohol at 0 - 50℃; for 4.5 - 5h; | 98.1% |
| In tert-butyl methyl ether; water; isopropyl alcohol at 50℃; for 1h; Product distribution / selectivity; | 98.1% |
| In tert-butyl methyl ether; water; isopropyl alcohol at 0 - 50℃; for 4h; Product distribution / selectivity; | 98.1% |

| Conditions | Yield |
|---|---|
| at 30℃; for 2h; | 98% |
| With sulfuric acid |
-
-
67-56-1
methanol

-
-
97-67-6
(S)-Malic acid

-
-
66212-45-1
(2S)-2-hydroxy-butanedioic acid 1-mono methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (S)-Malic acid With trifluoroacetic anhydride for 1.5h; Inert atmosphere; Stage #2: methanol for 2h; Inert atmosphere; regioselective reaction; | 98% |
| Stage #1: (S)-Malic acid With trifluoroacetic anhydride for 0.666667h; Stage #2: methanol at 20℃; for 1.5h; | 87% |
| Stage #1: (S)-Malic acid With (CF3CO2)2O at 20℃; Stage #2: methanol at 20℃; | 86% |
-
-
97-67-6
(S)-Malic acid

-
-
630-19-3
pivalaldehyde

-
-
56209-56-4
2-((2S,4S)-2-(tert-butyl)-5-oxo-1,3-dioxolan-4-yl)acetic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; toluene-4-sulfonic acid In pentane for 36h; Heating; | 98% |
| With sulfuric acid; toluene-4-sulfonic acid In pentane Heating; | 67% |
| With sulfuric acid In pentane Inert atmosphere; Reflux; | 50% |

| Conditions | Yield |
|---|---|
| With novozyme 435 at 70℃; under 15.0015 - 45.0045 Torr; for 48h; Enzymatic reaction; | 98% |


| Conditions | Yield |
|---|---|
| In methanol at 25 - 30℃; for 1h; | 98% |
| In methanol at 25 - 30℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| In methanol at 25 - 30℃; for 1h; | 98% |
| In methanol at 25 - 30℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| In methanol at 25 - 30℃; for 1h; | 98% |
| In methanol for 1h; | 98% |

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane at 20℃; for 8h; | 98% |
L(-)-Malic acid Chemical Properties
Structure of L-Malic acid (CAS NO.97-67-6):
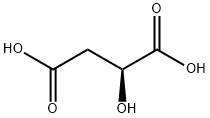
IUPAC Name: (2S)-2-hydroxybutanedioic acid
Empirical Formula: C4H6O5
Molecular Weight: 134.0874
EINECS: 202-601-5
Index of Refraction: 1.529
Molar Refractivity: 25.2 cm3
Molar Volume: 81.6 cm3
Polarizability: 9.99×10-24cm3
Surface Tension: 86.2 dyne/cm
Density: 1.641 g/cm3
Flash Point: 153.4 °C
Enthalpy of Vaporization: 63.43 kJ/mol
Melting Point: 101-103 °C(lit.)
Boiling Point: 306.4 °C at 760 mmHg
Vapour Pressure: 7.19E-05 mmHg at 25°C
Solubility: H2O: 0.5 M at 20 °C, clear, colorless
Water Solubility: soluble
Physical Appearance: clear colourless solution
Product Categories: Food & Feed ADDITIVES;MalicAcidSeries;Carboxylic Acids (Chiral);Chiral Building Blocks;for Resolution of Bases;Optical Resolution;Synthetic Organic Chemistry;Chiral chemicals
Product Name: L(-)-Malic acid
Synonyms of L-Malic acid (CAS NO.97-67-6): (-)-Hydroxysuccinic acid ; (-)-Malic acid ; (2S)-2-Hydroxybutanedioic acid ; 2-Hydroxybutanedioic acid, (S)- ; Apple acid ; Butanedioic acid, hydroxy-, (S)- ; Hydroxybutanedioic acid, (S)- ; L-(-)-Malic acid ; Malic acid, L- ; S-(-)-Malic acid ; S-2-Hydroxybutanedioic acid .
L(-)-Malic acid History
Malic acid was first isolated from apple juice by Carl Wilhelm Scheele in 1785. Antoine Lavoisier in 1787 proposed the name acide malique which is derived from the Latin word for apple, mālum.
L(-)-Malic acid Uses
L-Malic acid (CAS NO.97-67-6) is an active ingredient in many sour or tart foods.
L(-)-Malic acid Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany: 3
RTECS: ON7175000
HS Code: 29181980
L(-)-Malic acid Specification
Storage
It should be stored in a tightly closed container and keep container closed when not in use. It should be stored in a cool and dry area away from incompatible substances.
Handling
Wash thoroughly after handling.Avoid contact with eyes, skin, and clothing. Remove contaminated clothing and wash before reuse. Wash clothing before reuse. Avoid ingestion and inhalation. Use with adequate ventilation.
Personal Protection
Wear appropriate protective chemical safety goggles or eyeglasses. Wear appropriate protective gloves to prevent skin exposure.Wear suitable protective clothing to prevent skin exposure.
Small spills/leaks
Clean up spills right away, using the suitable protective equipment. Sweep up, then place into a container for disposal. Avoid generating dusty conditions. Provide ventilation.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View