-
Name
Methylglyoxal
- EINECS 201-164-8
- CAS No. 78-98-8
- Article Data342
- CAS DataBase
- Density 0.994 g/cm3
- Solubility >=10 g/100 mL at 17 ºC in water
- Melting Point 25 ºC
- Formula C3H4O2
- Boiling Point 71.999 ºC at 760 mmHg
- Molecular Weight 72.0636
- Flash Point 2.468 ºC
- Transport Information
- Appearance Clear yellow slightly viscous liquid with a pungent odor
- Safety 26-36
- Risk Codes 22-36
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Pyruvaldehyde(8CI);2-Ketopropionaldehyde;2-Oxopropanal;2-Oxopropionaldehyde;Acetylformaldehyde;Acetylformyl;NSC 626580;NSC 79019;Pyroracemic aldehyde;Pyruvic aldehyde;a-Ketopropionaldehyde;Methylglyoxal;
- PSA 34.14000
- LogP -0.22570
Synthetic route

| Conditions | Yield |
|---|---|
| With oxygen at 300℃; | A 5.79% B 92.17% C 2.04% |


| Conditions | Yield |
|---|---|
| With iron(III) phosphate; oxygen In water at 200℃; Oxidation; | 88% |
| With oxygen In methanol at 60℃; under 15001.5 Torr; for 6h; | 18% |
| With copper (I) acetate |

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 109.84℃; for 2h; Time; Reagent/catalyst; | 80% |

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide at 80℃; for 12h; | 74% |
| With sodium periodate; perchloric acid In water at 60℃; Rate constant; | |
| With ruthenium trichloride; sodium periodate; perchloric acid In water at 40 - 60℃; Thermodynamic data; Mechanism; ΔE(excit.), ΔH(excit.), ΔS(excit.); |

| Conditions | Yield |
|---|---|
| With methyl nitrite; nitrogen(II) oxide at 24.84℃; under 740 Torr; Inert atmosphere; Photolysis; | 65.2% |
| With ozone |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; vanadyl(IV) sulphate pentahydrate In water; acetonitrile at 20℃; for 6h; Green chemistry; | 65% |
| With air; silver contact at 390℃; | |
| With bromine; potassium carbonate |

| Conditions | Yield |
|---|---|
| at 400℃; under 0.01 Torr; for 2.77778E-06h; Kinetics; Temperature; | A 60% B 14% |
-
-
56-81-5
glycerol

-
A
-
2134-29-4
3-Hydroxypropanal

-
B
-
116-09-6
hydroxy-2-propanone

-
C
-
78-98-8
2-oxopropanal

| Conditions | Yield |
|---|---|
| In water at 400℃; | A 60% B 10% C 30% |


| Conditions | Yield |
|---|---|
| With methyl nitrite; nitrogen(II) oxide at 24.84℃; under 740 Torr; Inert atmosphere; Photolysis; | A 11.3% B 56.8% |
-
-
87-72-9, 608-45-7, 608-46-8, 608-47-9, 2460-44-8, 6748-95-4, 6763-34-4, 7261-26-9, 7283-06-9, 7283-07-0, 7296-55-1, 7296-56-2, 7296-58-4, 7296-59-5, 7296-60-8, 7296-61-9, 7296-62-0, 7322-30-7, 10257-31-5, 10257-32-6, 10257-33-7, 10257-34-8, 10257-35-9, 19982-83-3, 20242-88-0, 28697-53-2, 36562-42-2, 41546-41-2, 89299-64-9, 107655-34-5, 115794-06-4, 115794-07-5, 130550-15-1, 130606-21-2
D-Xylose

-
A
-
98-01-1
furfural

-
B
-
96-26-4
dihydroxyacetone

-
C
-
141-46-8
Glycolaldehyde

-
D
-
78-98-8
2-oxopropanal

| Conditions | Yield |
|---|---|
| With SO4(2-)/12 wt percent ZrO2-Al2O3/SBA-15 In water; toluene at 160℃; for 4h; | A 52.7% B n/a C n/a D n/a |


| Conditions | Yield |
|---|---|
| With sulfuric acid for 0.416667h; Heating; | 50% |
| With water Acidic conditions; | 50% |
| With sulfuric acid |
-
-
56-81-5
glycerol

-
A
-
64-18-6
formic acid

-
B
-
849585-22-4
LACTIC ACID

-
C
-
64-19-7
acetic acid

-
D
-
127-17-3
2-oxo-propionic acid

-
E
-
79-10-7
acrylic acid

-
F
-
78-98-8
2-oxopropanal

| Conditions | Yield |
|---|---|
| Stage #1: glycerol With sodium hydroxide; water at 300℃; for 1h; Compressed liquid(s); Stage #2: With sulfuric acid In water Product distribution / selectivity; | A n/a B 40% C n/a D n/a E n/a F n/a |


| Conditions | Yield |
|---|---|
| With tin(ll) chloride In water at 109.84℃; under 22502.3 Torr; for 3h; Catalytic behavior; Inert atmosphere; Autoclave; | A 5% B 39% |

| Conditions | Yield |
|---|---|
| With methyl nitrite; nitrogen(II) oxide at 24.84℃; under 740 Torr; Inert atmosphere; Photolysis; | A 38.5% B 17.8% |

| Conditions | Yield |
|---|---|
| With methyl nitrite; nitrogen(II) oxide at 24.84℃; under 740 Torr; Inert atmosphere; Photolysis; | A 13.4% B 34.5% |

| Conditions | Yield |
|---|---|
| With methyl nitrite; oxygen; nitrogen(II) oxide at 24.84℃; under 740 Torr; Inert atmosphere; Photolysis; | A 8.5% B 34.3% |

| Conditions | Yield |
|---|---|
| With MoO40W12(3-)*Al(3+) In water at 60℃; under 7500.75 Torr; for 20h; Autoclave; Inert atmosphere; | A 15% B 25% |
| With lutetium triflate In water at 109.84℃; for 1h; Reagent/catalyst; | |
| With silica-supported titanium oxide In water at 130℃; for 3h; Kinetics; Reagent/catalyst; | |
| In water at 90℃; for 4h; Catalytic behavior; Reagent/catalyst; Time; |

| Conditions | Yield |
|---|---|
| With MoO40W12(3-)*Al(3+); oxygen In water at 60℃; under 7500.75 Torr; for 20h; Kinetics; Autoclave; | A 25% B 14.5% C 6.2% |


| Conditions | Yield |
|---|---|
| With MoO40W12(3-)*Al(3+) In water at 60℃; under 7500.75 Torr; for 20h; Autoclave; Inert atmosphere; | A 25% B 9% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetonitrile at 20℃; under 760.051 Torr; for 5h; | A 24% B n/a |

| Conditions | Yield |
|---|---|
| With methyl nitrite; nitrogen(II) oxide at 24.84℃; under 740 Torr; Inert atmosphere; Photolysis; | A 23.7% B 18.6% |

| Conditions | Yield |
|---|---|
| With serpentine at 300 - 445℃; Flow reactor; | A 5.6% B 10.1% C 22.4% D 6.2% |
| With serpentine at 300 - 500℃; Flow reactor; | A 13.9% B 18% C 16.7% D 8.7% |
| With sulfated zirconia with MgO at 300 - 500℃; Flow reactor; | A 6.2% B 12.5% C 14.3% D 5.4% |


| Conditions | Yield |
|---|---|
| With sulfated zirconia at 300 - 445℃; Flow reactor; | A 19.6% B 5.2% |
| With sulfated zirconia with MgO at 300 - 445℃; Flow reactor; | A 17.8% B 6.6% |
| With silica-alumina with MgO at 300 - 445℃; Flow reactor; | A 5.2% B 5.3% |

| Conditions | Yield |
|---|---|
| With methyl nitrite; nitrogen(II) oxide at 24.84℃; under 740 Torr; Inert atmosphere; Photolysis; | A 6.4% B 18.1% |

| Conditions | Yield |
|---|---|
| With 3H(1+)*MoO40W12(3-); oxygen In water at 60℃; under 7500.75 Torr; for 20h; Autoclave; | A 16% B 14.9% C 8.4% |


| Conditions | Yield |
|---|---|
| With silica-alumina at 300 - 445℃; Flow reactor; | A 9.4% B 9.4% C 6% |


| Conditions | Yield |
|---|---|
| With methyl nitrite; nitrogen(II) oxide at 24.84℃; under 740 Torr; Inert atmosphere; Photolysis; | 8.4% |
-
-
14920-89-9
2,3-dimethylfuran

-
A
-
535-09-1
3-methyl-4-oxo-2-pentenal

-
B
-
131543-46-9
Glyoxal

-
C
-
141-46-8
Glycolaldehyde

-
D
-
431-03-8
dimethylglyoxal

-
E
-
78-98-8
2-oxopropanal

| Conditions | Yield |
|---|---|
| With hydroxyl radical; nitrogen(II) oxide at 22.84℃; under 735 Torr; Gas phase; | A 8% B n/a C n/a D n/a E n/a |

-
-
110-86-1
pyridine

-
-
1620-14-0
1-(diethylamino)propan-2-one

-
-
94-36-0
dibenzoyl peroxide

-
A
-
109-89-7
diethylamine

-
B
-
78-98-8
2-oxopropanal


| Conditions | Yield |
|---|---|
| With tetrachloromethane; ozone |
-
-
78-98-8
2-oxopropanal

-
-
849585-22-4
LACTIC ACID

| Conditions | Yield |
|---|---|
| With barium hydroxide octahydrate at 25℃; for 48h; Inert atmosphere; | 100% |
| With scandium(III) chloride In water at 109.84℃; for 1h; Reagent/catalyst; | 90% |
| With aluminum (III) chloride In water at 170℃; for 0.333333h; Autoclave; | 87% |
-
-
27298-98-2
(S)-1-(4-methylphenyl)ethylamine

-
-
78-98-8
2-oxopropanal

| Conditions | Yield |
|---|---|
| 100% |

| Conditions | Yield |
|---|---|
| 100% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In ethanol; water at 20℃; for 12h; | 100% |
| With sodium hydrogencarbonate In ethanol; water at 20℃; for 18h; Inert atmosphere; | |
| With sodium hydrogencarbonate In ethanol; water | |
| With sodium hydrogencarbonate In ethanol; water at 20℃; for 18h; |

| Conditions | Yield |
|---|---|
| With acetic acid In water at 20℃; | 100% |
| In water at 25℃; Inert atmosphere; | 85% |
| With acetic acid In water at 20℃; for 1h; |
-
-
1073-62-7, 20570-96-1
N-benzylhydrazine hydrochloride

-
-
78-98-8
2-oxopropanal

-
-
109299-37-8
1-benzylhydrazonopropanone

| Conditions | Yield |
|---|---|
| In water at 20℃; | 100% |
-
-
60-34-4
methylhydrazine

-
-
78-98-8
2-oxopropanal

-
-
85985-64-4
1-(4-hydroxy-1,5-dimethyl-1H-pyrazol-3-yl)ethanone

| Conditions | Yield |
|---|---|
| With acetic acid In water for 3h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In water | 100% |

| Conditions | Yield |
|---|---|
| In methanol; water at 0 - 20℃; for 2.75h; | 99% |

| Conditions | Yield |
|---|---|
| In water at 80℃; for 5h; | 99% |


| Conditions | Yield |
|---|---|
| In water at 80℃; for 5h; | 99% |


| Conditions | Yield |
|---|---|
| In water at 20℃; for 1.5h; | 98% |
| In water at 20℃; for 2h; | 85% |
| Stage #1: tertbutylhydrazine hydrochloride; 2-oxopropanal In water at 23℃; Stage #2: With sodium hydroxide In tert-butyl methyl ether; water |

| Conditions | Yield |
|---|---|
| In water at 80℃; for 5h; Solvent; Temperature; Time; | 98% |
| With water In neat (no solvent) for 0.1h; Microwave irradiation; Green chemistry; |


| Conditions | Yield |
|---|---|
| In water at 80℃; for 5h; | 98% |


| Conditions | Yield |
|---|---|
| In water at 80℃; for 5h; | 98% |


| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 37℃; for 2h; Darkness; | 98% |

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 37℃; for 24h; | A 98% B n/a C n/a D n/a E n/a |

-
-
4426-21-5
oxybis(diphenylborane)

-
-
1670-14-0
benzamidine monohydrochloride

-
-
78-98-8
2-oxopropanal

-
-
115438-37-4
1-methyl-3,3,7-triphenyl-2,4-dioxa-8-aza-6-azonia-3-boratabicyclo{3.3.3}oct-6-ene

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol room temp.; crystn. from reaction mixt., washing (ethanol, ether), or evapn., extraction (hot ethanol), crystn. on cooling; elem. anal.; | 97% |

-
-
146896-52-8
2-phenyl-5-methylene-4,5-dihydrooxazole

-
-
78-98-8
2-oxopropanal

-
-
1616778-00-7
3-hydroxy-4-(2-phenyloxazol-5-yl)butan-2-one

| Conditions | Yield |
|---|---|
| With C39H60N4O4*Ni(2+)*2BF4(1-)*6H2O In dichloromethane at 30℃; for 24h; enantioselective reaction; | 97% |
-
-
343338-28-3
(S)-2-methylpropane-2-sulfinamide

-
-
78-98-8
2-oxopropanal

| Conditions | Yield |
|---|---|
| With copper(II) sulfate In dichloromethane at 23℃; for 24h; Inert atmosphere; | 97% |
-
-
6340-91-6
n-propylhydrazine oxalate

-
-
78-98-8
2-oxopropanal

-
-
1355228-90-8
2-oxopropanal propylhydrazone

| Conditions | Yield |
|---|---|
| Stage #1: n-propylhydrazine oxalate; 2-oxopropanal With acetic acid In water at 20℃; Stage #2: With sodium hydrogencarbonate In dichloromethane; water | 96% |

| Conditions | Yield |
|---|---|
| With sodium sulfate In diethyl ether; water at 20℃; for 24h; | 96% |

| Conditions | Yield |
|---|---|
| With acetic acid In water | 96% |

| Conditions | Yield |
|---|---|
| In water at 80℃; for 5h; | 96% |


| Conditions | Yield |
|---|---|
| In water at 80℃; for 5h; | 96% |


| Conditions | Yield |
|---|---|
| With 1-hydroxyethylene-(1,1-diphosphonic acid) In water at 80℃; for 1h; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| In water for 0.0333333h; Isay condensation; microwave irradiation; | 95% |
| Stage #1: 2-oxopropanal With sodium disulfite In water for 0.166667h; Addition; Stage #2: 1,2-diamino-benzene In water at 20℃; for 18h; Condensation; Cyclization; | 91% |
| In water at 20℃; for 0.5h; Green chemistry; | 85% |

| Conditions | Yield |
|---|---|
| With sodium acetate In water for 0.5h; Heating; | 95% |
| Stage #1: semicarbazide hydrochloride With sodium acetate In ethanol for 0.333333h; Reflux; Stage #2: 2-oxopropanal for 0.583333h; Reflux; | 89% |
| With water; potassium acetate |

| Conditions | Yield |
|---|---|
| Heating; | 95% |
| In water for 1.5h; Reflux; | 90% |
| In water | 87% |
-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
-
78-98-8
2-oxopropanal

| Conditions | Yield |
|---|---|
| With C32H29N3O3S In water at 25℃; for 30h; Aldol Addition; enantioselective reaction; | A 95% B n/a |

Methylglyoxal Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 51 ,1991,p. 443.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 51 ,1991,p. 443.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human No Available Data IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 51 ,1991,p. 443.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Reported in EPA TSCA Inventory.
Methylglyoxal Specification
The Methylglyoxal, with the CAS registry number 78-98-8, is also known as Acetylformaldehyde. It belongs to the product categories of Aldehydes; Blocks; Building Blocks; Aliphatics. Its EINECS registry number is 201-164-8. This chemical's molecular formula is C3H4O2 and molecular weight is 72.06. What's more, both its IUPAC name and systematic name are the same which is called 2-Oxopropanal. It is the aldehyde form of pyruvic acid. It has two carbonyl groups, so it is a dicarbonyl compound. In organisms, this chemical is formed as a side-product of several metabolic pathways. It may form from 3-amino acetone, which is an intermediate of threonine catabolism, as well as through lipid peroxidation. However, the most important source is glycolysis.
Physical properties about Methylglyoxalare:
(1) # of Rule of 5 Violations: 0; (2)ACD/BCF (pH 5.5): 1; (3)ACD/BCF (pH 7.4): 1; (4)ACD/KOC (pH 5.5): 14.181; (5)ACD/KOC (pH 7.4): 14.181; (6)#H bond acceptors: 2; (7)#H bond donors: 0; (8)#Freely Rotating Bonds: 1; (9)Polar Surface Area: 34.14 Å2; (10)Index of Refraction: 1.364; (11)Molar Refractivity: 16.164 cm3; (12)Molar Volume: 72.494 cm3; (13)Surface Tension: 27.133 dyne/cm; (14)Density: 0.994 g/cm3; (15)Flash Point: 2.468 °C; (16)Enthalpy of Vaporization: 31.343 kJ/mol; (17)Boiling Point: 71.999 °C at 760 mmHg; (18)Vapour Pressure: 120.93 mmHg at 25 °C.
Preparation of Methylglyoxal:
Methylglyoxal can be prepared by 1-Hydroxy-propan-2-one. This reaction needs reagents O2, FePO4 and solvent H2O at temperature of 200 °C. The yield is 88 %.

Uses of Methylglyoxal:
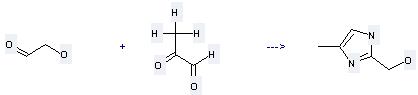
(1) it is used as medicine, pesticide intermediates and biochemical reagents; (2) it is used to produce other chemicals. For example, it can react with Hydroxyacetaldehyde to get (4-Methyl-1(3)H-imidazol-2-yl)-methanol. The reaction occurs with reagent NH3 and solvent ethanol at temperature of 40 °C . The yield is 35 %.
Safety Information of Methylglyoxal:
When you are dealing with Methylglyoxal, you should be very careful. This chemical may cause damage to health and cause inflammation to the skin or other mucous membranes. It is irritating to eyes and it is harmful if swallowed. Therefore, you should wear suitable protective clothing. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: CC(=O)C=O
(2) InChI: InChI=1S/C3H4O2/c1-3(5)2-4/h2H,1H3
(3) InChIKey: AIJULSRZWUXGPQ-UHFFFAOYSA-N
The toxicity data of Methylglyoxal is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 179mg/kg (179mg/kg) | National Cancer Institute Screening Program Data Summary, Developmental Therapeutics Program. Vol. JAN1986, | |
| mouse | LD50 | intravenous | 252mg/kg (252mg/kg) | Cesko-Slovenska Farmacie. Vol. 15, Pg. 300, 1966. | |
| rat | LD50 | oral | 1165mg/kg (1165mg/kg) | Ecotoxicology and Environmental Safety. Vol. 2, Pg. 369, 1978. |
Related Products
- Methylglyoxal
- Methylglyoxal 1,1-dimethyl acetal
- 789-96-8
- 79002-39-4
- 79002-96-3
- 79-00-5
- 79005-83-7
- 79009-37-3
- 79-01-6
- 790184-33-7
- 790227-48-4
- 79025-97-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View