-
Name
Nitrogen trifluoride
- EINECS 232-007-1
- CAS No. 7783-54-2
- Article Data107
- CAS DataBase
- Density 1.361 g/cm3
- Solubility Solubility in water 0.021 vol/vol (20 °C, 1 bar)
- Melting Point ?206.8 °C (66.35 K)
- Formula F3N
- Boiling Point -129 ºC
- Molecular Weight 71.0019
- Flash Point
- Transport Information UN 2451
- Appearance colorless gas
- Safety 17-23-38
- Risk Codes 8-20
-
Molecular Structure
-
Hazard Symbols
 O
O
- Synonyms Nitrogentrifluoride; Perfluoroammonia; Trifluoroamine; Trifluoroammonia
- PSA 3.24000
- LogP 0.94190
Synthetic route
-
-
400090-26-8
(Cl2Te)2N(1+)*GaCl4(1-)=[(Cl2Te)2N]GaCl4

-
-
7784-36-3
arsenic pentafluoride

-
A
-
7783-54-2
nitrogen trifluoride

| Conditions | Yield |
|---|---|
| In liquid sulphur dioxide Ga-compd.:AsF5 molar ratio was 1:2.5, stirring for 12 h at 20 °C; soln. was concd. in vac., crystn. on cooling, drying for 12 h. in vac. at 20 °C, elem. anal.; NF3 was detected by IR; | A n/a B 75% |

-
-
10036-47-2
tetrafluorohydrazine

-
-
201230-82-2
carbon monoxide

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
10578-16-2
dinitrogen difluoride

-
C
-
353-50-4
Carbonyl fluoride

-
D
-
2368-32-3
trifluoromethylamide

| Conditions | Yield |
|---|---|
| byproducts: N2O, CO2, SiF4; other Radiation; ratio of N2F4 and CO = 1:2, irradiation by high pressure Hg lamp for 2h at room temp.; fractionated distn.; | A n/a B n/a C n/a D 15% |
| byproducts: N2O, CO2, SiF4; other Radiation; ratio of N2F4 and CO = 1:2, irradiation by high pressure Hg lamp for 2h at room temp.; fractionated distn.; | A n/a B n/a C n/a D 15% |


-
-
75-02-5
1-fluoroethylene

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
74-90-8
hydrogen cyanide

-
C
-
7664-39-3
hydrogen fluoride

-
D
-
7664-41-7
ammonia

| Conditions | Yield |
|---|---|
| byproducts: CH3CN, C2H2F2, CH2CN; in a Pyrex tube at 0.95 Torr; products were detected by mass spectrometry; |


| Conditions | Yield |
|---|---|
| In hydrogen fluoride Electrolysis; in anhydrous HF at -10°C; |
-
-
10036-47-2
tetrafluorohydrazine

-
A
-
7783-54-2
nitrogen trifluoride

-
C
-
10544-72-6
dinitrogen tetraoxide

-
D
-
7789-25-5
nitrosyl fluoride

| Conditions | Yield |
|---|---|
| In neat (no solvent) 60°C, 18 h;; |


-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
7727-37-9
nitrogen

-
C
-
1333-74-0
hydrogen

-
D
-
10028-15-6
ozone

-
E
-
10024-97-2
dinitrogen monoxide

| Conditions | Yield |
|---|---|
| In melt byproducts: O2; Electrolysis; reaction by electrolysis of molten NH4F*HF with a Cu cathode and a graphite anode at 125 °C;; | |
| In melt byproducts: O2; Electrolysis; reaction by electrolysis of molten NH4F*HF with a Cu cathode and a graphite anode at 125 °C;; |


| Conditions | Yield |
|---|---|
| byproducts: N2, HF; violent reaction; further unidenfed products formed; | |
| In neat (no solvent) | |
| In neat (no solvent) | |
| byproducts: N2, HF; violent reaction; further unidenfed products formed; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) quenching reaction at 1500-4000K;; |

-
-
7664-39-3
hydrogen fluoride

-
-
113-00-8
guanidine nitrate

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
75-73-0
carbon tetrafluoride

-
C
-
7727-37-9
nitrogen

| Conditions | Yield |
|---|---|
| In hydrogen fluoride Electrolysis; at 15°C; ratio of NF3:CF4 = 2.5; 40 % soln. of guanidine in anhydrous liquid HF; | |
| In hydrogen fluoride HF (liquid); Electrolysis; at 15°C; ratio of NF3:CF4 = 2.5; 40 % soln. of guanidine in anhydrous liquid HF; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) | |
| In neat (no solvent) | >99 |
| In neat (no solvent) | |
| In neat (no solvent) | >99 |
-
-
7664-39-3
hydrogen fluoride

-
-
4426-72-6, 51433-48-8
hydrazine carboxamide

-
A
-
10036-47-2
tetrafluorohydrazine

-
B
-
7783-54-2
nitrogen trifluoride

-
C
-
75-73-0
carbon tetrafluoride

-
D
-
353-50-4
Carbonyl fluoride

| Conditions | Yield |
|---|---|
| In hydrogen fluoride byproducts: CO2, N2, C; Electrolysis; 1 M soln. of semicarbazide in anhydrous liquid HF; 5.6-5.7 V; ratio of NF3 : CF4 depends on potential used; | A 0% B n/a C n/a D n/a |
| In hydrogen fluoride byproducts: CO2, N2, C; HF (liquid); Electrolysis; 1 M soln. of semicarbazide in anhydrous liquid HF; 5.6-5.7 V; ratio of NF3 : CF4 depends on potential used; | A 0% B n/a C n/a D n/a |

-
-
10036-47-2
tetrafluorohydrazine

-
-
373-91-1
hypofluorous acid trifluoromethyl ester

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
75-73-0
carbon tetrafluoride

-
C
-
353-50-4
Carbonyl fluoride

-
D
-
4217-93-0
N,N-Difluor-O-trifluormethylhydroxylamin

| Conditions | Yield |
|---|---|
| Irradiation (UV/VIS); ambient temp.; |

-
-
13773-81-4
krypton difluoride

-
-
335-01-3
difluoramino trifluoromethane

-
-
7784-36-3
arsenic pentafluoride

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
13776-62-0
trans-1,2-difluorodiazine

| Conditions | Yield |
|---|---|
| With HF In hydrogen fluoride byproducts: CF4; HF (liquid); reagents were mixed at -196°C for 3 h; |


| Conditions | Yield |
|---|---|
| In hydrogen fluoride Electrolysis; |
-
-
1512-14-7
S,S-difluoro-N-trifluoromethyl-sulfimide

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
75-73-0
carbon tetrafluoride

-
C
-
335-01-3
difluoramino trifluoromethane

-
D
-
359-62-6
fluoro-bis-trifluoromethyl-amine

-
E
-
2551-62-4
sulfur(VI) hexafluoride

| Conditions | Yield |
|---|---|
| Electrochem. Process; electrochemical fluorination, further product: CHF3; | |
| Electrochem. Process; electrochemical fluorination, further product: CHF3; |


| Conditions | Yield |
|---|---|
| In hydrogen fluoride Electrolysis; |
-
-
4101-37-5
pentafluoroethyliminosulfur difluoride

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
75-73-0
carbon tetrafluoride

-
C
-
76-16-4
Hexafluoroethane

-
D
-
354-80-3
Perfluoroethylamine

-
E
-
2551-62-4
sulfur(VI) hexafluoride

| Conditions | Yield |
|---|---|
| Electrochem. Process; electrochemical fluorination, further products: C2F5H and CHF3; | |
| Electrochem. Process; electrochemical fluorination, further products: C2F5H and CHF3; |

-
-
7783-41-7
difluoroether

-
-
7789-25-5
nitrosyl fluoride

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
80937-33-3
oxygen

| Conditions | Yield |
|---|---|
| In neat (no solvent) NOF is passed into OF2;; | |
| In neat (no solvent) NOF is slowly passed into a vessel filled with OF2; inflammation;; | |
| In neat (no solvent) NOF is passed into OF2;; | |
| In neat (no solvent) NOF is slowly passed into a vessel filled with OF2; inflammation;; |


-
-
7783-54-2
nitrogen trifluoride

| Conditions | Yield |
|---|---|
| In melt byproducts: N2, O2, N2F2; Electrolysis; 25 mA/cm**2, 100°C; further byproducts; XRD; SEM; XPS; | |
| In melt byproducts: N2, N2F2, H2; Electrolysis; electrolysis of molten NH4F*2HF with and without LiF conducted at 25 mA/cm**2 for 120 h with Ni or nickel-nickel oxide composite anodes; electrolyte kept at 100°C; |

| Conditions | Yield |
|---|---|
| In melt F2 and molten NH4F*2HF at 90°C;; |
-
-
460-19-5
ethanedinitrile

-
-
7727-37-9
nitrogen

-
-
7782-41-4
fluorine

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
75-73-0
carbon tetrafluoride

-
C
-
76-16-4
Hexafluoroethane

-
D
-
335-01-3
difluoramino trifluoromethane

-
E
-
359-62-6
fluoro-bis-trifluoromethyl-amine

| Conditions | Yield |
|---|---|
| further products; by heating to 175-250°C; | |
| further products; by heating to 175-250°C; |

-
-
10036-47-2
tetrafluorohydrazine

-
-
460-19-5
ethanedinitrile

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
75-73-0
carbon tetrafluoride

-
C
-
354-80-3
Perfluoroethylamine

-
D
-
5131-88-4
N,N-Difluor-cyanodifluormethylamin

-
E
-
1426-41-1
N,N,N',N'-Tetrafluor-1,2-diamino-1,1,2,2-tetrafluor-ethan

-
-
16871-76-4
tetrafluoroammonium hexafluoroantimonate

-
-
177570-08-0, 7789-30-2, 676353-69-8
bromine pentafluoride

-
-
65391-03-9
Cs(1+)*BrOF4(1-)=CsBrOF4

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
16949-12-5
cesium hexafluoroantimonate

| Conditions | Yield |
|---|---|
| byproducts: BrF3O; addn. at -196°C, stirred at 20°C for 2.5 h, cooled to -196°C; the noncondensable material distd. off, the voleatile material sept. byfractional distn., the solid nonvolatile product determined by Raman spetr.; |


| Conditions | Yield |
|---|---|
| In water Electrolysis; no product but evolution of H2 (cathode) and N2/O2 (anode);; | A 0% B n/a C n/a D n/a |
| In water Electrolysis; no product but evolution of H2 (cathode) and N2/O2 (anode);; | A 0% B n/a C n/a D n/a |


-
-
7783-54-2
nitrogen trifluoride

| Conditions | Yield |
|---|---|
| In water Electrolysis; concd. soln.;; | |
| In water Electrolysis; concd. soln.;; |

-
-
7782-41-4
fluorine

-
A
-
10405-27-3
difluoroamine

-
B
-
7783-54-2
nitrogen trifluoride

-
C
-
3744-07-8
difluoroamino radical

-
D
-
13967-06-1
N-monofluoroamine

-
-
10036-47-2
tetrafluorohydrazine

-
-
7790-91-2
chlorine trifluoride

-
A
-
7783-54-2
nitrogen trifluoride

-
B
-
7790-89-8
chlorine monofluoride

| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; at 180 - 210°C in Al tube;; |


| Conditions | Yield |
|---|---|
| 100 Torr PuF6, 2250 Torr N2, room temp., 229 d; | |
| 100 Torr PuF6, 2250 Torr N2, room temp., 229 d; |

| Conditions | Yield |
|---|---|
| In hydrogen fluoride Electrolysis; electrolysis of piperidine in liq. HF;; fractional distn.;; | |
| In hydrogen fluoride Electrolysis; electrolysis of piperidine in liq. HF;; fractional distn.;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) plasma react. in inductively coupled tubular reactor, temp. of reactor exterior <=180°C, 200 mg of powdered precursor in alumina boat, power of 150-200 W, chamber pressure of 250 mTorr, NF3 flow rate of 10 sccm, reaction time of 10-20 min; X-ray diffraction anal.; EDX anal.; chem. anal.; | 99% |
-
-
7783-54-2
nitrogen trifluoride

-
-
7783-70-2
antimony pentafluoride

-
-
10024-97-2
dinitrogen monoxide

-
-
12528-55-1
NF2O(1+)*Sb2F11(1-) =(NF2O)(Sb2F11)

| Conditions | Yield |
|---|---|
| byproducts: N2; 150°C; | 99% |
| 100°C; | 0% |

-
-
7783-54-2
nitrogen trifluoride

-
-
13773-81-4
krypton difluoride

-
-
7784-36-3
arsenic pentafluoride

-
-
16871-75-3
NF4(1+)*AsF6(1-)

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: Kr; mixt. of KrF2, AsF5 and NF3 (molar ratio 2:1:7) was loaded in stainlesssteel cylinder at -196°C, warmed slowly to room temp. over 30 h,kept at 53°C for 4 d, cooled to -210°C on N2 slush bath; volatiles sepd. by fractional condensation through traps at -156 and -210°C; identified by IR and Raman spectra; | 96.7% |

-
-
7783-54-2
nitrogen trifluoride

-
-
201230-82-2
carbon monoxide

-
A
-
75-73-0
carbon tetrafluoride

-
B
-
353-50-4
Carbonyl fluoride

-
C
-
10102-43-9
nitrogen(II) oxide

-
D
-
7789-25-5
nitrosyl fluoride

-
E
-
10102-44-0
Nitrogen dioxide

| Conditions | Yield |
|---|---|
| nickel tube, equimolar amt., 825°C; | A n/a B 80% C n/a D n/a E n/a |

-
-
7783-54-2
nitrogen trifluoride

-
-
371-71-1
Perfluoro-2-azapropen

-
-
686-39-5
tris-trifluoromethyl-carbamimidoyl fluoride

| Conditions | Yield |
|---|---|
| 550°C, in presence of CsF; | 41.7% |
| 550°C, in presence of CsF; | 41.7% |
-
-
7783-54-2
nitrogen trifluoride

-
-
506-77-4
cyanogen chloride

-
A
-
675-14-9
trifluoro-[1,3,5]triazine

-
B
-
73513-59-4
cis-hexafluoroazomethane

-
C
-
371-71-1
Perfluoro-2-azapropen

| Conditions | Yield |
|---|---|
| In not given at 500°C, in Ni-tube, in excess of NF3; | A 40% B 30% C 30% |

-
-
7783-54-2
nitrogen trifluoride

-
-
13773-81-4
krypton difluoride

-
-
7637-07-2
boron trifluoride

-
-
15640-93-4
tetrafluoroammonium tetrafluoroborate

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: Kr; KrF2, BF3 and NF3 (molar ratio 2:1:7) was loaded in stainless steel cylinder at -196°C, warmed slowly to room temp. over 30 h, kept at 53°C for 4 d, cooled to -210°C on N2 slush bath; volatiles sepd. by fractional condensation through traps at -156 and -210°C; | 30.6% |
| In hydrogen fluoride KrF2, BF3 and NF3 was loaded into cylinder at -196°C, warmed slowly to 25°C, kept at 25°C for 3 h; | 28.1% |
| In hydrogen fluoride KrF2, BF3 and NF3 was loaded into cylinder at -196°C, warmed slowly to -78°C, kept at -78°C for 3 h; | 7.1% |

-
-
7783-54-2
nitrogen trifluoride

-
-
7446-11-9
sulfur trioxide

-
A
-
640723-20-2, 2699-79-8, 12769-73-2
fluorosulfonyl fluoride

-
C
-
7783-42-8
thionyl fluoride

-
D
-
2551-62-4
sulfur(VI) hexafluoride

| Conditions | Yield |
|---|---|
| byproducts: NO, NO2; 520 °C, nickel tube; | A 30% B n/a C 4% D <1 |


| Conditions | Yield |
|---|---|
| at 500°C; | 30% |
| at 500°C; | 30% |

| Conditions | Yield |
|---|---|
| 500°C, in a flow system (contact time=0.6 s); | 30% |
| 500°C, in a flow system (contact time=0.6 s); | 30% |

| Conditions | Yield |
|---|---|
| >1500°C; | 16% |
-
-
7783-54-2
nitrogen trifluoride

-
-
371-71-1
Perfluoro-2-azapropen

-
-
13400-13-0
cesium fluoride

-
-
338-66-9
perfluoromethanimine

| Conditions | Yield |
|---|---|
| by pyrolysis over CsF at 540 to 560°C; | 6.7% |
| by pyrolysis over CsF at 540 to 560°C; | 6.7% |

-
-
7783-54-2
nitrogen trifluoride

-
-
7637-07-2
boron trifluoride

-
-
7782-41-4
fluorine

-
-
15640-93-4
tetrafluoroammonium tetrafluoroborate

| Conditions | Yield |
|---|---|
| In neat (no solvent) Irradiation (UV/VIS); equivalent mixture of NF3, F2 and BF3, 298K, 6.5atm;; | 1% |
| In neat (no solvent) Irradiation (UV/VIS); equivalent mixture of NF3, F2 and BF3, 298K, 6.5atm;; | 1% |
| In neat (no solvent) 5.6mmol BF3, 6.1mmol NF3, contineous react. with F2 at 30Torr and 0.4l/h, 5kV, 30mA, 20min; heating to 60°C, removal of volatile components in vac.;; heating to 200.degreee.C in vac. for 30-40min;; | >99 |

-
-
7783-54-2
nitrogen trifluoride

-
-
7446-11-9
sulfur trioxide

-
A
-
640723-20-2, 2699-79-8, 12769-73-2
fluorosulfonyl fluoride

-
B
-
2551-62-4
sulfur(VI) hexafluoride

| Conditions | Yield |
|---|---|
| under pressure, 230-440 °C, molar ratio 1:1; |


| Conditions | Yield |
|---|---|
| under pressure, 230-440 °C, molar ratio of NF3:SO3 = 2:1; |

| Conditions | Yield |
|---|---|
| byproducts: N2; at red heat (with fire); |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: Ba3N2; heating of Ba with NF3 at approx. 200°C under formation of BaF2 with some Ba3N2;; |

| Conditions | Yield |
|---|---|
| byproducts: Bi fluoride; thermic reaction;; | |
| byproducts: Bi fluoride; thermic reaction;; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; etching of SiO2 film on Si wafer by NF3/Ar mixt.; detd. by FTIR; | |
| In neat (no solvent) calcination of SiO2 and NF3;; | |
| In neat (no solvent) calcination of SiO2 and NF3;; |
-
-
7783-54-2
nitrogen trifluoride

-
-
7440-67-7
zirconium(IV) oxide

-
-
851363-60-5, 7783-64-4
zirconium(IV) fluoride

| Conditions | Yield |
|---|---|
| sample fluorination at 250 and 300°C for 4 h; XRD; |
NITROGEN TRIFLUORIDE Chemical Properties
NITROGEN TRIFLUORIDE(7783-54-2) is the chemical compound with the formula F3N .Its molar weight is 71.
NITROGEN TRIFLUORIDE(7783-54-2) chemical structural formula is
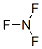
Synonyms:N,N,N-Trifluoroamine;NF3;Nitrogen fluoride (NF3);nitrogenfluoride(nf3);Perfluoroammonia;Stickstoff(III)-fluorid;Stickstofftrifluorid;Trifluoroamine
mp: -207 ℃
bp: -129 ℃
Water Solubility: insoluble
CAS :7783-54-2
NITROGEN TRIFLUORIDE Uses
NITROGEN TRIFLUORIDE(7783-54-2)is used in the plasma etching of silicon wafers. Today it is mainly employed in the cleaning of the PECVD chambers in the high volume production of liquid crystal displays and silicon-based thin film solar cells. In these applications NF3 is initially broken down in situ, by a plasma. The resulting fluorine atoms are the active cleaning agents that attack the polysilicon,silicon nitride and silicon oxide. Nitrogen trifluoride can be used as well with tungsten silicide, and tungsten produced by CVD. Erroneously NF3 has been considered as an environmentally preferable substitute for perfluorocarbons such as hexafluoroethane,sulfur hexafluoride etc. The process utilization of the chemicals applied in plasma processes is typically below 20 %. Therefore some of the PFCs and also of the NF3 always escape into the atmosphere. Modern gas abatement systems can decrease such emissions.
Recently elemental fluorine has been introduced as an environmentally friendly replacement for nitrogen trifluoride in state-of-the-art high volume manufacturing of flat panel displays and solar cell manufacturing.
NITROGEN TRIFLUORIDE(7783-54-2) is also used in hydrogen fluoride and deuterium fluoride lasers, which are types of chemical lasers. It is preferred to fluorine gas due to its convenient handling properties, reflecting its considerable stability.
It is compatible with steel and Monel, as well as several plastics.
NITROGEN TRIFLUORIDE Production
Today, it is prepared both by direct reaction of ammonia and fluorine and by a variation of Ruff's method.It is supplied in pressurized cylinders.
NITROGEN TRIFLUORIDE Toxicity Data With Reference
| 1. | ihl-rat LC50:6700 ppm/1H | TXAPA9 Toxicology and Applied Pharmacology. 26 (1973),1. | ||
| 2. | ihl-mus LC50:2000 ppm/4H | 34ZIAG Toxicology of Drugs and Chemicals ,Deichmann, W.B.,New York, NY.: Academic Press, Inc.,1969,427. | ||
| 3. | ihl-dog LC50:9600 ppm/1H | AMRL** Aerospace Medical Research Laboratory Report. (Aerospace Technical Div., Air Force Systems Command, Wright-Patterson Air Force Base, OH 45433) TR-69-84 ,1969. | ||
| 4. | ihl-mky LC50:7500 ppm/1H | AMRL** Aerospace Medical Research Laboratory Report. (Aerospace Technical Div., Air Force Systems Command, Wright-Patterson Air Force Base, OH 45433) TR-69-84 ,1969. |
NITROGEN TRIFLUORIDE Consensus Reports
NITROGEN TRIFLUORIDE Safety Profile
NITROGEN TRIFLUORIDE Standards and Recommendations
ACGIH TLV: TWA 10 ppm; BEI: 3 mg/g creatinine of fluorides in urine prior to shift; 10 mg/g creatinine of fluorides in urine at end of shift.
NIOSH REL: (Inorganic Fluorides) TWA 2.5 mg(F)/m3
DOT Classification: 2.2; Label: Nonflammable Gas, Oxidizer
NITROGEN TRIFLUORIDE Specification
Related Products
- Nitrogen
- Nitrogen chloride
- Nitrogen dioxide
- Nitrogen Fluoride Oxide
- Nitrogen monoxide, mixed with nitrogen tetroxide
- Nitrogen oxide (N2O5)
- NITROGEN TETROXIDE
- NITROGEN TRIFLUORIDE
- Nitrogen triiodide
- 77835-49-5
- 7783-56-4
- 7783-58-6
- 7783-60-0
- 7783-61-1
- 7783-63-3
- 7783-64-4
- 7783-66-6
- 7783-70-2
- 7783-71-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View