-
Name
Trimethylsilyl cyanide
- EINECS 231-657-3
- CAS No. 7677-24-9
- Article Data62
- CAS DataBase
- Density 0.802 g/cm3
- Solubility reacts with water
- Melting Point 8-11 °C(lit.)
- Formula C4H9NSi
- Boiling Point 118.499 °C at 760 mmHg
- Molecular Weight 99.2077
- Flash Point 1.111 °C
- Transport Information UN 3384 6.1/PG 1
- Appearance clear colorless to yellow liquid
- Safety 16-36/37/39-45-61-26
- Risk Codes 11-26/27/28-29-50/53
-
Molecular Structure
-
Hazard Symbols
 F,
F, T+,
T+, T,
T, N
N
- Synonyms Silane,cyanotrimethyl- (6CI);Cyanotrimethylsilane;Trimethylcyanosilane;Trimethylsilanecarbonitrile;Trimethylsilyl nitrile;Trimethylsilylcarbonitrile;Trimethylsilycyanide;
- PSA 23.79000
- LogP 1.38738
Synthetic route

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one at 200℃; | 96% |
| at 170 - 200℃; | 89% |
-
-
2857-97-8
trimethylsilyl bromide

-
-
65300-07-4
bis(triphenylphosphine)iminium cyanide

-
-
7677-24-9
trimethylsilyl cyanide

| Conditions | Yield |
|---|---|
| at 20℃; for 1h; | 93% |

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; potassium iodide for 12h; Ambient temperature; | 88% |
| With PEG400; zinc(II) iodide In dichloromethane at 20℃; for 20h; | 86% |
| With 1-methyl-pyrrolidin-2-one; potassium iodide for 72h; Ambient temperature; | 78% |
-
-
3012-37-1
benzyl thiocyanate

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
351-60-0
benzyl trifluoromethyl sulfide

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A n/a B 87% |


| Conditions | Yield |
|---|---|
| With PEG400; zinc(II) iodide In dichloromethane at 20℃; for 16h; | 83% |
| With PEG400; zinc(II) iodide In dichloromethane at 20℃; for 4h; sonification; | 80% |
| With sodium iodide solvent: N-methyl-2-pyrrolidone; | 70% |
| With 1-methyl-pyrrolidin-2-one; AMBERLITE XAD-4 for 0.05h; Ambient temperature; |
-
-
16029-98-4
trimethylsilyl iodide

-
A
-
7677-24-9
trimethylsilyl cyanide

| Conditions | Yield |
|---|---|
| In benzene at 65℃; for 36h; | A n/a B 81% |

-
-
623-26-7
terephthalonitrile

-
-
18292-38-1
2-methallyltrimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
97780-97-7
4-(2-methyl-2-propenyl)benzonitrile

| Conditions | Yield |
|---|---|
| In acetonitrile Irradiation; | A n/a B 80% |

-
-
19942-78-0
octyl thiocyanate

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
134776-65-1
n-octyll trifluoromethyl sulfide

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A n/a B 76% |

-
-
542-90-5
ethyl isothiocyanate

-
-
13716-45-5
diethyl trimethylsilyl phosphite

-
A
-
1186-09-0
O,O,S-triethyl phosphorothioate

-
B
-
7677-24-9
trimethylsilyl cyanide

| Conditions | Yield |
|---|---|
| at 100 - 110℃; for 1h; | A 75% B n/a |
| at 100 - 110℃; for 1h; Yields of byproduct given; |

-
-
81328-78-1
1-octaneselenocyanate

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A n/a B 75% |


| Conditions | Yield |
|---|---|
| for 15h; Heating; | 72% |
-
-
556-64-9
methyl thiocyanate

-
-
24350-54-7
diisopropyl trimethylsily phosphite

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
22907-64-8
O,O-diisopropyl S-methyl phosphorothioate

| Conditions | Yield |
|---|---|
| at 100 - 110℃; for 1h; | A n/a B 70% |
| at 100 - 110℃; for 1h; Yields of byproduct given; |

-
-
5285-87-0
phenyl thiocyanate

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
456-56-4
phenyl trifluoromethylsulfide

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A n/a B 70% |

-
-
4671-93-6
benzyl selenocyanate

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
186047-38-1
{[(trifluoromethyl)selanyl]methyl}benzene

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A n/a B 70% |


| Conditions | Yield |
|---|---|
| 68% |
-
-
91-15-6
phthalonitrile

-
-
762-72-1
allyl-trimethyl-silane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
61463-61-4
2-allylbenzonitrile

| Conditions | Yield |
|---|---|
| In acetonitrile Irradiation; | A n/a B 67% |

-
-
623-26-7
terephthalonitrile

-
-
762-72-1
allyl-trimethyl-silane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
51980-05-3
4-allylbenzonitrile

| Conditions | Yield |
|---|---|
| In acetonitrile Irradiation; | A n/a B 66% |

-
-
625-59-2
2-propyl thiocyanate

-
-
24350-54-7
diisopropyl trimethylsily phosphite

-
A
-
63785-58-0
O,O,S-triisopropyl phosphorothioate

-
B
-
7677-24-9
trimethylsilyl cyanide

| Conditions | Yield |
|---|---|
| at 100 - 110℃; for 1h; | A 64% B n/a |
| at 100 - 110℃; for 1h; Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With AMBERLITE XAD-4; sodium cyanide In various solvent(s) at 25℃; for 0.5h; | 61% |
| With NaCN impregnated on Amberlite XAD-4 In acetonitrile at 60℃; for 1.5h; Product distribution; effect of other reagent, other solvents, reaction time and temperature; | 95 % Chromat. |
-
-
2179-79-5
phenyl selenocyanate

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
5173-02-4
phenyl trifluoromethylselenide

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A n/a B 58% |

-
-
2137-92-0
4-nitrophenyl thiocyanate

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
403-66-7
p-nitrophenyl trifluoromethyl sulfide

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A n/a B 58% |

-
-
18292-38-1
2-methallyltrimethylsilane

-
-
91-15-6
phthalonitrile

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
97780-98-8
2-(2-methyl-2-propenyl)benzonitrile

| Conditions | Yield |
|---|---|
| In acetonitrile Irradiation; | A n/a B 44% |


| Conditions | Yield |
|---|---|
| 150 to 200 deg C; | 42% |
-
-
4251-13-2
thiocyanatocyclohexane

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
6476-52-4
cyclohexyl(trifluoromethyl)sulfane

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A n/a B 33% |

-
-
18519-25-0
1-methyl-1H-pyrrol-2-yl thiocyanate

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
58679-52-0
1-methyl-2-trifluoromethylsulfanyl-pyrrole

-
B
-
7677-24-9
trimethylsilyl cyanide

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A 32% B n/a |

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
A
-
7677-24-9
trimethylsilyl cyanide

-
B
-
66476-29-7
2,4-dimethoxy-1-[(trifluoromethyl)sulfanyl]benzene

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran 0 deg C, 5 min then rt., 2.5 h; | A n/a B 30% |


| Conditions | Yield |
|---|---|
| With lithium | |
| (i) LiH, ether, (ii) /BRN= 1209232/; Multistep reaction; |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
108-94-1
cyclohexanone

-
-
24731-36-0
1-trimethylsilanyloxycyclohexanecarbonitrile

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one at 20℃; for 4h; Inert atmosphere; | 100% |
| With MgAlCO3-HT In n-heptane for 0.0833333h; Ambient temperature; | 99% |
| With tris(2-hydroxyethyl)amine-N-oxide; N-benzyl-N,N-dimethylphenylmethylammonium bromide In dichloromethane at 23℃; for 16h; | 99% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
555-16-8
4-nitrobenzaldehdye

-
-
71189-80-5
2-(4-nitrophenyl)-2-[(trimethylsilyl)oxy]acetonitrile

| Conditions | Yield |
|---|---|
| With 2Zn(2+)*10H2O*3C10H8N2*2H(1+)*Co2Mo10H4O38(6-) In neat (no solvent) at 25℃; for 7h; Reagent/catalyst; Inert atmosphere; | 100% |
| With C42H50Mg2N4 In benzene-d6 at 25℃; Glovebox; Inert atmosphere; | 99% |
| With cobalt incorporated covalent organic framework of 2,3-dihydroxyterephthalohydrazide with triformylphloroglucinol (NUS-51-Co) In dichloromethane at 24.84℃; for 10h; Catalytic behavior; | 99% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
100-52-7
benzaldehyde

-
-
25438-37-3
phenyl(trimethylsiloxy)acetonitrile

| Conditions | Yield |
|---|---|
| tris(bis(trimethylsilyl)amido)samarium(III) In tetrahydrofuran at 0℃; for 4h; | 100% |
| With lithium tetrafluoroborate In acetonitrile at 20℃; for 2h; | 100% |
| iron(III) chloride In nitromethane at 0℃; for 0.5h; | 100% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
75-07-0
acetaldehyde

-
-
41309-99-3
2-<(trimethylsilyl)oxy>propanenitrile

| Conditions | Yield |
|---|---|
| With Zr(IV) metal-organic framework with 1,4-benzenedicarboxylate in anhydrous form In dichloromethane at 40℃; for 46h; Inert atmosphere; Reflux; | 100% |
| With Eu2(benzene-1,2,3,4,5,6-hexacarboxylate)(H2O)3 In acetonitrile at 20 - 100℃; for 3h; | 99% |
| With Mn(5-((4-(tetrazol-5-yl)benzyl)oxy)isophthalic acid)2(H2O)2 In neat (no solvent) at 20℃; Catalytic behavior; Reagent/catalyst; | 99% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
123-38-6
propionaldehyde

-
-
24731-32-6
2-<(trimethylsilyl)oxy>butanenitrile

| Conditions | Yield |
|---|---|
| With trans-{(iBu)2ATIGeiPr}2Pt(CN)2 In chloroform-d1 at 50℃; for 2h; Catalytic behavior; Schlenk technique; Glovebox; | 100% |
| With C29H38AlN4O2(1+)*CF3O3S(1-) at 20℃; for 0.0833333h; Catalytic behavior; Inert atmosphere; Schlenk technique; | 99% |
| With Mn(5-((4-(tetrazol-5-yl)benzyl)oxy)isophthalic acid)2(H2O)2 In neat (no solvent) at 20℃; Catalytic behavior; Reagent/catalyst; | 99% |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane at 20℃; for 72h; Inert atmosphere; | 100% |
| for 168h; Ambient temperature; | 63% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
830-13-7
cyclododecanone

-
-
50361-51-8
1-trimethylsilyloxy-1-cyclododecanecarbonitrile

| Conditions | Yield |
|---|---|
| With zinc(II) iodide | 100% |
| With lithium perchlorate; tetraethylammonium perchlorate In dichloromethane Ambient temperature; electrolysis: 0.28 F/mol; | 95% |
| With n-butyllithium In tetrahydrofuran; hexane for 2h; Ambient temperature; | 90.6% |
| With zinc(II) iodide |
-
-
6974-32-9
1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose

-
-
7677-24-9
trimethylsilyl cyanide

-
-
23316-67-8
2,3,5-tri-O-benzoyl-β-D-ribofuranosyl-1-carbonitrile

| Conditions | Yield |
|---|---|
| With tin(IV) chloride | 100% |
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 20℃; stereoselective reaction; | 100% |
| With boron trifluoride diethyl etherate In nitromethane Ambient temperature; | 87% |

| Conditions | Yield |
|---|---|
| With isopropoxydiisobutylaluminum In hexane Product distribution; Ambient temperature; other oxiranes; var. Lewis acids; var. solvents; var. temperatures; | 100% |
| With aluminum isopropoxide 1.) RT, 2 h, 2.) RT, 15 h; Yield given. Multistep reaction; |
-
-
503-30-0
trimethylene oxide

-
-
7677-24-9
trimethylsilyl cyanide

-
-
72049-81-1
4-<(trimethylsilyl)oxy>butyronitrile

| Conditions | Yield |
|---|---|
| With diethylaluminium chloride for 18h; Ambient temperature; | 100% |
-
-
98-01-1
furfural

-
-
7677-24-9
trimethylsilyl cyanide

-
-
40861-56-1
2-(2-furyl)-2-(trimethylsilyloxy)acetonitrile

| Conditions | Yield |
|---|---|
| With Eu2(benzene-1,2,3,4,5,6-hexacarboxylate)(H2O)3 In acetonitrile at 20 - 100℃; for 1h; | 100% |
| With 1-methoxy-2-methyl-1-(trimethylsiloxy)propene at 19℃; for 10h; | 99% |
| With potassium phtalimide at 20℃; for 1.16667h; solvent-free; | 99% |
-
-
1003-67-4
4-methylpyridine-1-oxide

-
-
7677-24-9
trimethylsilyl cyanide

-
-
1620-76-4
2-Cyano-4-methylpyridin

| Conditions | Yield |
|---|---|
| With N,N-Dimethylcarbamoyl chloride In dichloromethane for 120h; Ambient temperature; | 100% |
| Stage #1: 4-methylpyridine-1-oxide; trimethylsilyl cyanide With N,N-Dimethylcarbamoyl chloride In dichloromethane at 20℃; for 24h; Stage #2: With potassium carbonate In dichloromethane; water for 0.5h; | 96% |
| With triethylamine In acetonitrile at 100 - 110℃; for 20h; | 89% |
-
-
931-19-1
2-methylpyridine N-oxide

-
-
7677-24-9
trimethylsilyl cyanide

-
-
1620-75-3
2-Cyano-6-methylpyridine

| Conditions | Yield |
|---|---|
| With N,N-Dimethylcarbamoyl chloride In dichloromethane for 120h; Ambient temperature; | 100% |
| With dimethylcarbamide chloride In dichloromethane at 20℃; for 24h; | 98% |
| With N,N-Dimethylcarbamoyl chloride In Nitroethane at 20℃; for 22h; | 28% |
-
-
563-80-4
3-methyl-butan-2-one

-
-
7677-24-9
trimethylsilyl cyanide

-
-
883726-88-3
2,3-dimethyl-2-((trimethylsilyl)oxy)butanenitrile

| Conditions | Yield |
|---|---|
| With trans-{(iBu)2ATIGeiPr}2Pt(CN)2 In chloroform-d1 at 50℃; for 6h; Catalytic behavior; Schlenk technique; Glovebox; | 100% |
| With C29H38AlN4O2(1+)*CF3O3S(1-) In neat (no solvent) at 20℃; for 0.0833333h; Catalytic behavior; Inert atmosphere; Schlenk technique; | 99% |
| With C48H40N8Sm(1-)*C16H32LiO4(1+) at 20℃; for 4h; Inert atmosphere; Schlenk technique; | 99% |
-
-
14371-10-9
(E)-3-phenylpropenal

-
-
7677-24-9
trimethylsilyl cyanide

-
-
100573-50-0, 40326-21-4, 79248-45-6
(E)-4-phenyl-2-(trimethylsiloxy)but-3-enenitrile

| Conditions | Yield |
|---|---|
| With zinc(II) iodide In dichloromethane at 20℃; for 2h; Addition; | 100% |
| With 1-methoxy-2-methyl-1-(trimethylsiloxy)propene at 19℃; for 36h; | 99% |
| With tin-tungsten mixed oxide, Sn/W molar ratio = 2, calcined at 800 °C In 1,2-dichloro-ethane at 22 - 23℃; for 1h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane | 100% |

-
-
95-16-9
1,3-Benzothiazole

-
-
7677-24-9
trimethylsilyl cyanide

-
-
98-88-4
benzoyl chloride

-
-
95479-51-9
3-benzoyl-2,3-dihydro-2-benzothiazolecarbonitrile

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane other aroyl chlorides; | 100% |
| With aluminium trichloride In dichloromethane | 100% |
| With aluminium trichloride In dichloromethane for 72h; Ambient temperature; | 50% |
| With aluminium trichloride In dichloromethane | 44% |

-
-
95-16-9
1,3-Benzothiazole

-
-
7677-24-9
trimethylsilyl cyanide

-
-
874-60-2
4-methyl-benzoyl chloride

-
-
95479-50-8
2,3-dihydro-3-(4-methylbenzoyl)-2-benzothiazolecarbonitrile

| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane | 100% |
| With aluminium trichloride In dichloromethane for 72h; Ambient temperature; | 87% |
| With aluminium trichloride In dichloromethane for 72h; Ambient temperature; | 86% |


| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane | 100% |


| Conditions | Yield |
|---|---|
| With aluminium trichloride In dichloromethane | 100% |

-
-
23569-17-7
4-tert-butylpyridine N-oxide

-
-
7677-24-9
trimethylsilyl cyanide

-
-
42205-73-2
4-tert-butyl-2-cyanopyridine

| Conditions | Yield |
|---|---|
| With N,N-Dimethylcarbamoyl chloride In dichloromethane at 20℃; | 100% |
| With triethylamine In acetonitrile at 100℃; for 25h; | 97% |
| With N,N-Dimethylcarbamoyl chloride In dichloromethane for 24h; Ambient temperature; | |
| With N,N-Dimethylcarbamoyl chloride In dichloromethane cyanidation; |
-
-
924-44-7
glyoxylic acid ethyl ester

-
-
7677-24-9
trimethylsilyl cyanide

-
-
137622-41-4
(+/-)-ethyl 2-cyano-2-hydroxyacetate

| Conditions | Yield |
|---|---|
| With sodium acetate buffer In ethanol; water for 0.166667h; Ambient temperature; | 100% |
| With sodium acetate; acetic acid In ethanol for 0.166667h; Ambient temperature; | 1.29 g |
| Stage #1: glyoxylic acid ethyl ester With copper(II) bis(trifluoromethanesulfonate) In dichloromethane at 20℃; for 0.25h; Stage #2: trimethylsilyl cyanide In dichloromethane at 20℃; for 18h; |

| Conditions | Yield |
|---|---|
| In dichloromethane for 18h; | 100% |

-
-
99-49-0, 22327-39-5
Carvone

-
-
7677-24-9
trimethylsilyl cyanide

-
-
75983-86-7
5-Isopropenyl-2-methyl-3-trimethylsilanyloxy-cyclohex-2-enecarbonitrile

| Conditions | Yield |
|---|---|
| With triethylaluminum In tetrahydrofuran for 6h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With [Sc3(3,5-disulfobenzoic acid)2(μ-O2H3)(μ-OH)2(H2O)2] In neat (no solvent) at 40℃; for 2h; Catalytic behavior; Schlenk technique; Inert atmosphere; | 100% |
| With 2Zn(2+)*10H2O*3C10H8N2*2H(1+)*Co2Mo10H4O38(6-) In neat (no solvent) at 25℃; for 7h; Reagent/catalyst; Inert atmosphere; | 100% |
| With tin-tungsten mixed oxide, Sn/W molar ratio = 2, calcined at 800 °C In 1,2-dichloro-ethane at 22 - 23℃; for 0.5h; Inert atmosphere; | 99% |
-
-
111-26-2
hexan-1-amine

-
-
7677-24-9
trimethylsilyl cyanide

-
-
87367-65-5
4,4'-[decane-1,10-diylbis(oxy)]dibenzaldehyde

-
-
145100-47-6
(4-{10-[4-(Cyano-hexylamino-methyl)-phenoxy]-decyloxy}-phenyl)-hexylamino-acetonitrile

| Conditions | Yield |
|---|---|
| In dichloromethane for 120h; | 100% |

-
-
7677-24-9
trimethylsilyl cyanide

-
-
34780-29-5
3-oxo-propionic acid ethyl ester

-
-
132839-91-9
(+/-)-ethyl 3-cyano-3-hydroxypropionate

| Conditions | Yield |
|---|---|
| With sodium hydroxide; acetic acid In ethanol; water | 100% |
| With sodium acetate buffer In ethanol; water for 1h; Ambient temperature; | 98% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
2094-74-8
1-Adamantanecarbaldehyde

-
-
79671-70-8
adamantan-1-yltrimethylsilanyloxy-acetonitrile

| Conditions | Yield |
|---|---|
| With zinc(II) iodide | 100% |
| With zinc(II) iodide In dichloromethane at 0℃; for 1h; | 75.3% |
| With zinc(II) iodide at 20℃; for 150h; | |
| With zinc(II) iodide In chloroform for 1h; |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
16473-11-3
bicyclo[3.3.1]nonane-2,6-dione

-
-
75993-72-5
2,6-Bis(trimethylsilyloxy)bicyclo<3.3.1>nonan-2,6-dicarbonitril

| Conditions | Yield |
|---|---|
| With zinc(II) iodide In dichloromethane at 40℃; for 0.5h; | 100% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
33892-75-0
5-Methoxy-1-tetralone

-
-
124921-27-3
1-cyano-1-(trimethylsilyloxy)-5-methoxy-1,2,3,4-tetrahydronaphthalene

| Conditions | Yield |
|---|---|
| With zinc(II) iodide In benzene at 25℃; for 3h; | 100% |
| With zinc(II) iodide for 48h; Ambient temperature; | |
| With zinc(II) iodide |
Trimethylsilyl cyanide Specification
The Silanecarbonitrile,trimethyl-, with the CAS registry number 7677-24-9, is also known as Cyanotrimethylsilane. It belongs to the product categories of Small molecule; Si (Classes of Silicon Compounds); Si-(C)4 Compounds; Silicon Compounds (for Synthesis); Synthetic Organic Chemistry. Its EINECS number is 231-657-3. This chemical's molecular formula is C4H9NSi and molecular weight is 99.21. What's more, its systematic name is trimethylsilanecarbonitrile. It is sentive to moisture, and it is a kind of flammable liquid so that it should be sealed and stored in containers with dry inert gas at the temperature of 0 - 6 °C. Moreover, it should be protected from oxides, acids and water. This chemical is prepared by the reaction of lithium cyanide and trimethylsilyl chloride. It is used in organic synthesis as the equivalent of hydrogen cyanide.
Physical properties of Silanecarbonitrile,trimethyl- are: (1)ACD/LogP: 1.27; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.267; (4)ACD/LogD (pH 7.4): 1.267; (5)ACD/BCF (pH 5.5): 5.404; (6)ACD/BCF (pH 7.4): 5.404; (7)ACD/KOC (pH 5.5): 116.432; (8)ACD/KOC (pH 7.4): 116.432; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 23.79 Å2; (13)Index of Refraction: 1.388; (14)Molar Refractivity: 29.211 cm3; (15)Molar Volume: 123.682 cm3; (16)Polarizability: 11.58×10-24cm3; (17)Surface Tension: 19.154 dyne/cm; (18)Density: 0.802 g/cm3; (19)Flash Point: 1.111 °C; (20)Enthalpy of Vaporization: 35.672 kJ/mol; (21)Boiling Point: 118.499 °C at 760 mmHg; (22)Vapour Pressure: 16.624 mmHg at 25°C.
Preparation: this chemical can be prepared by potassium cyanide and trimethyl chlorosilicane by heating. The reaction time is 16 hours. The yield is about 71%.
(CH3)3SiCl + KCN → (CH3)3SiCN + KCl
Uses of Silanecarbonitrile,trimethyl-: it can be used to produce 2-phenyl-2-trimethylsilanyloxy-propionitrile at the temperature of 20 °C. It will need reagent InBr3 and solvent CH2Cl2 with the reaction time of 3 hours. The yield is about 90%.
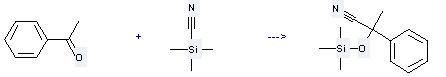
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable, so you should keep it away from sources of ignition - No smoking. It is very toxic by inhalation, in contact with skin and if swallowed. When contacted with water, it will react violently with water and liberate toxic gas. It is very toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible). You should avoid releasing it to the environment just refering to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)InChI: InChI=1S/C4H9NSi/c1-6(2,3)4-5/h1-3H3
(2)InChIKey: LEIMLDGFXIOXMT-UHFFFAOYSA-N
(3)Canonical SMILES: C[Si](C)(C)C#N
Related Products
- Trimethylsilyl 2-(fluorosulfonyl)difluoroacetate
- Trimethylsilyl acetate
- Trimethylsilyl bromoacetate
- Trimethylsilyl chlorosulphate
- Trimethylsilyl cyanide
- Trimethylsilyl isobutyrate
- Trimethylsilyl methacrylate
- Trimethylsilyl methanesulfonate
- Trimethylsilyl perchlorate
- Trimethylsilyl propionate
- 76777-25-8
- 76780-96-6
- 76782-82-6
- 76783-59-0
- 76787-63-8
- 76-78-8
- 76788-46-0
- 76792-22-8
- 76792-94-4
- 76795-95-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View