-
Name
p-Tolualdehyde
- EINECS 203-246-9
- CAS No. 104-87-0
- Article Data1436
- CAS DataBase
- Density 1.015 g/cm3
- Solubility 0.25 g/L (25 ºC)
- Melting Point -6 °C
- Formula C8H8O
- Boiling Point 204.5 °C at 760 mmHg
- Molecular Weight 120.151
- Flash Point 80 °C
- Transport Information
- Appearance clear colorless to pale yellow liquid
- Safety 26-36/37/39
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms para-Toluyl aldehyde;p-Methyl benzaldehyde;4-Methyl benzaldehyde;p-tolualdehyde;4-methylBenzaldehyde;4-Toluicaldehyde;p-Formyltoluene;FEMA No. 3068;4-Toluylaldehyde;4-Methylbenzaldehyde;Benzaldehyde, 4-methyl-;Methylbenzaldehyde information;
- PSA 17.07000
- LogP 1.80750
Synthetic route

| Conditions | Yield |
|---|---|
| With dinitrogen tetraoxide; ferric nitrate In dichloromethane Ambient temperature; further oxidizing agent, further conditions and solvents; | 100% |
| In ethanol at 40℃; for 9h; | 100% |
| With TGSE; sodium hydrogencarbonate; sodium carbonate In water for 1.66667h; Electrochemical reaction; | 100% |

| Conditions | Yield |
|---|---|
| With oxygen; 10-methyl-9-phenylacridin-10-ium perchlorate In chloroform at 24.84℃; for 10h; Oxidation; Pyrolysis; visible light; | 100% |
| With sulfuric acid; 9-mesityl-2,7,10-trimethylacridinium perchlorate; water; oxygen In acetonitrile at 24.84℃; for 1.33333h; Quantum yield; Reagent/catalyst; Irradiation; | 100% |
| With N-hydroxyphthalimide; oxygen; cobalt(II) acetate; acetic acid at 20℃; under 760.051 Torr; chemoselective reaction; | 100% |
-
-
120445-48-9, 3030-95-3
4-methylbenzaldehyde semicarbazone

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride; Tonsil In ethyl acetate for 2h; Heating; | 100% |
| With copper(II) sulfate In tetrahydrofuran; methanol; water for 48h; Heating; | 95% |
| With potassium permanganate; montmorillonite K-10 for 0.25h; | 94% |

| Conditions | Yield |
|---|---|
| With [NO(1+)*18-crown-6*H(NO3)2(1-)]; silica gel In dichloromethane at 20℃; for 0.0833333h; | 100% |
| With sulphated zirconia In acetonitrile at 60℃; for 0.3h; Microwave irradiation; | 100% |
| With Montmorillonite K10 In dichloromethane for 0.333333h; Heating; | 99% |
-
-
40739-81-9
p-tolualdehyde p-toluenesulfonylhydrazone

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With copper(II) sulfate In tetrahydrofuran; methanol; water for 48h; Heating; | 100% |
| With Cr-MCM-41 zeolite on silica gel for 0.15h; microwave irradiation; | 98% |
| Amberlyst 15 In water; acetone at 80℃; for 24h; | 86% |
| With polymer-supported phenyliodine(III) diacetate; water In dichloromethane at 20℃; for 2h; Oxidation; | 78% |

| Conditions | Yield |
|---|---|
| With copper(II) sulfate In tetrahydrofuran; methanol; water for 6.5h; Heating; | 100% |
| With benzyltriphenylphosphonium peroxodisulfate In acetonitrile for 0.133333h; Heating; | 95% |
| With CuCl*Kieselghur; oxygen In dichloromethane at 20℃; for 0.5h; | 94% |
-
-
160594-72-9
N,N-diethyl-4-methylselenobenzamide

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 0℃; for 3h; | 100% |
-
-
89200-92-0
trimethyl(4-methylbenzyloxy)silane

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With nitrogen dioxide at 20℃; for 0.0833333h; | 100% |
| With phosphomolybdic acid In toluene for 0.166667h; Heating; | 97% |
| With allyltriphenylphopsphonium peroxodisulfate In acetonitrile for 0.25h; Heating; | 96% |

| Conditions | Yield |
|---|---|
| With sodium periodate; C31H29Br2N3Ru*CH2Cl2 In water; ethyl acetate; acetonitrile at 25℃; for 0.5h; Inert atmosphere; Schlenk technique; | 100% |
| With tert.-butylhydroperoxide; manganese(II,III) oxide In acetonitrile at 70℃; for 3h; | 98% |
| With iron(III) trifluoromethanesulfonate; 2-((4R,5R)-1-((4-(tert-butyl)phenyl)sulfonyl)-4,5-diphenylimidazolidin-2-yl)-6-((4R,5R)-1-((4-(tert-butyl)phenyl)sulfonyl)-4,5-diphenylimidazolidin-2-yl)pyridine; oxygen In 1,2-dichloro-ethane at 70℃; under 760.051 Torr; for 6h; Reagent/catalyst; Solvent; Green chemistry; chemoselective reaction; | 96% |
-
-
1203650-44-5
N-(2-hydroxyl-1-(2-pyridyl)-2-(4-methylphenyl)ethyl)benzenecarbothioamide

-
A
-
1203650-52-5
bis(3-phenyl-1-imidazo[1,5-a]pyridyl)-4-methylphenylmethane

-
B
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With pyridine; iodine In tetrahydrofuran at 20℃; for 0.5h; | A 100% B n/a |

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With α,α-diphenyl-2-pyrrolidinemethanol trimethylsilyl ether In chloroform at 20℃; for 24h; regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 24.85℃; under 56886.3 Torr; for 0.5h; Product distribution; Further Variations:; Pressures; Temperatures; | 99.1% |
| With 1,10-Phenanthroline; triphenylphosphine; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate; scandium tris(trifluoromethanesulfonate); 1-n-butyl-3-methylimidazolium hexafluoroantimonate; cerium triflate at 50℃; under 15001.5 Torr; for 5h; Reagent/catalyst; Autoclave; Inert atmosphere; | 76.8% |
| With trifluorormethanesulfonic acid under 95000.1 Torr; for 4h; Ambient temperature; | 72 % Chromat. |

| Conditions | Yield |
|---|---|
| Stage #1: 4-methylbenzoic acid ethyl ester With morpholine; diisobutylaluminium hydride In tetrahydrofuran; hexane at 0℃; for 3.16667h; Inert atmosphere; Stage #2: With diisobutylaluminium hydride In tetrahydrofuran; hexane at 0℃; for 0.166667h; Inert atmosphere; | 99% |
| With sodium tris(diethylamino)aluminum hydride In tetrahydrofuran; dodecane at 0℃; for 24h; | 93% |
| With phenylsilane; cobalt(II) diacetate tetrahydrate; sodium triethylborohydride In 1,2-dimethoxyethane; toluene at 25℃; for 15h; Inert atmosphere; Schlenk technique; | 92% |
-
-
51490-06-3
1,2-di-p-tolyl-ethanone

-
-
62-53-3
aniline

-
A
-
6833-18-7
4-methyl-N-phenylbenzamide

-
B
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; copper(II) chloride dihydrate; oxygen In acetonitrile under 760.051 Torr; for 24h; Reflux; | A 99% B n/a |

-
-
106-42-3
para-xylene

-
A
-
100-21-0
terephthalic acid

-
B
-
104-87-0
4-methyl-benzaldehyde

-
C
-
619-66-9
4-Carboxybenzaldehyde

-
D
-
99-94-5
p-Toluic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide; oxygen; acetic acid; cobalt(II) acetate; manganese(II) acetate In water at 190℃; under 16501.7 Torr; for 1h; Product distribution / selectivity; | A 98.1% B 0.2% C 0.4% D 0.4% |
| With oxygen; acetic acid; palladium diacetate; antimony(III) acetate In water at 182 - 195℃; under 16501.7 - 20929.4 Torr; for 1 - 1.5h; Product distribution / selectivity; | A 50.3% B 7.2% C 6.4% D 6.2% |
| With hydrogen bromide; oxygen; acetic acid; zirconium oxyacetate; cobalt(II) acetate In water at 190℃; under 16501.7 Torr; for 1h; Product distribution / selectivity; | A 4.9% B 3% C 1.9% D 36.9% |


| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 20℃; for 0.05h; Microwave irradiation; | 98% |
| With 4-methylmorpholine N-oxide; 1-ethyl-3-methyl-1H-imidazol-3-ium chloride; potassium iodide at 100℃; for 0.0333333h; Microwave irradiation; Ionic liquid; | 94% |
| With 1-dodecyl-3-methylimidazolium iron chloride; periodic acid at 30℃; for 1.5h; | 93% |
-
-
3235-02-7, 3717-15-5, 3717-16-6, 140461-26-3, 140461-27-4
p-methylbenzaldehyde oxime

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With CuCl*Kieselghur; oxygen In dichloromethane at 20℃; for 0.333333h; | 98% |
| Stage #1: p-methylbenzaldehyde oxime With hexachlorodisilane; silica gel In toluene at 110℃; for 0.5h; Stage #2: With water In toluene for 0.5h; | 97% |
| In dichloromethane at 20℃; for 4h; | 96% |
-
-
18484-04-3
2-((4-methylbenzyl)oxy)tetrahydro-2H-pyran

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With zeofen (zeolite HZSM-5, Fe(NO3)3*9H2O) In dichloromethane for 6h; Heating; | 98% |
| With chromium(VI) oxide; HZSM-5 zeolite for 0.0333333h; microwave irradiation; | 95% |
| With NTPPPODS In acetonitrile for 0.166667h; Reflux; | 94% |
-
-
79439-44-4
C16H18OSe

-
A
-
13527-05-4
4-methylbenzyl nitrate

-
B
-
66361-14-6
p-methylbenzeneseleninic acid

-
C
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With nitric acid In dichloromethane at -5 - 0℃; for 1h; | A 98% B n/a C n/a D n/a E n/a |
| With nitric acid In dichloromethane at -5 - 0℃; for 1h; | A n/a B 98% C n/a D n/a E n/a |


| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; N-methyl-N-[3-(4-diacetoxyiodo)phenoxy-1-propyl]pyrrolidinium 4-methylbenzenesulfonate In dichloromethane at 20℃; for 1.5h; Inert atmosphere; | 98% |

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 0.0416667h; Product distribution; Ambient temperature; pH = 4-6, regeneration of aldehyde; | 97.1% |

| Conditions | Yield |
|---|---|
| With lithium diisobutylmorpholinoaluminum hydride In tetrahydrofuran; hexane at 0℃; for 0.5h; | 97% |
| With calcium bis(hypophosphite); calcium acetate; nickel(II) acetate tetrahydrate In ethanol; water at 100℃; for 7h; Sealed tube; | 94% |
| With C13H26B(1-)*K(1+) In tetrahydrofuran for 24h; Ambient temperature; | 83% |

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 20℃; for 0.0666667h; Microwave irradiation; | 97% |
| With dihydrogen peroxide In ethanol for 4h; Reflux; Green chemistry; | 95% |
| With 1-dodecyl-3-methylimidazolium iron chloride; periodic acid at 30℃; for 1.5h; | 93% |
-
-
71778-40-0
1-((phenylthio)(p-tolyl)methylthio)benzene

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With sodium perborate In acetic acid at 25℃; for 2h; | 97% |
| With oxygen; 2,4,6-tris(p-chlorophenyl)pyrylium perchlorate In dichloromethane for 0.2h; Irradiation; | 83% |

| Conditions | Yield |
|---|---|
| With potassium permanganate; Amberlite IR-120; water In dichloromethane at 20℃; for 0.333333h; | 97% |
| With sulfuric acid; quinolinium dichromate(VI) In acetic acid at 29.9℃; Rate constant; Thermodynamic data; ΔHact, ΔSact, ΔGact; var. concentration of reagents; | |
| Multi-step reaction with 2 steps 1: magnesium chloride; ATP / 20 h / pH 7.5 / Enzymatic reaction 2: water / 14 h / 30 °C / Enzymatic reaction View Scheme |
-
-
122334-36-5
N-methoxy-N,4-dimethylbenzamide

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With lithium diisobutyl-tert-butoxyaluminum hydride In tetrahydrofuran; hexane at 0℃; Reagent/catalyst; Inert atmosphere; chemoselective reaction; | 97% |
| With benzoic acid ethyl ester; copper diisobutyl-t-butoxyaluminum hydride In tetrahydrofuran at 20℃; for 12h; Inert atmosphere; chemoselective reaction; | 96 %Chromat. |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; tetrakis(pyridine)silver(II) peroxodisulfate; oxygen In N,N-dimethyl-formamide at 23℃; under 760.051 Torr; Irradiation; | 97% |

| Conditions | Yield |
|---|---|
| With polyaniline-sulfate salt; water for 0.5h; Heating; | 96% |
| With silica-OSO3H; silica gel In toluene at 60 - 70℃; for 1h; | 94% |
| In(OSO2CF3)3 In acetone at 20℃; for 4h; | 92% |
-
-
23229-29-0
2-(4-methylphenyl)-1,3-dithiolane

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With tetrachlorosilane; dimethyl sulfoxide In dichloromethane at 20℃; for 0.583333h; | 96% |
| With silica gel In neat (no solvent) at 20℃; for 0.05h; | 96% |
| With sodium nitrate; sulfuric acid; silica gel; 4-nitrobenzylidene diacetate In dichloromethane at 20℃; for 0.25h; | 93% |

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In acetonitrile at 40℃; for 0.5h; Irradiation; | A 96% B n/a |
| With ammonium cerium(IV) nitrate In acetonitrile at 40℃; for 0.5h; Mechanism; Irradiation; | A 96% B n/a |
| ammonium cerium(IV) nitrate; sodium dodecyl-sulfate In water at 25℃; for 3h; | A 3.95 mmol B 3.10 mmol |
| With ammonium cerium(IV) nitrate; sodium dodecyl-sulfate In water at 25℃; for 3h; Title compound not separated from byproducts; | A 3.95 mmol B 3.10 mmol |
| With sodium dodecyl-sulfate; ammonium cerium(IV) nitrate In water at 25℃; for 3h; Product distribution; other reagents, solvents, reac. time; | A 3.95 mmol B 3.1 mmol |
-
-
79-24-3
Nitroethane

-
-
104-87-0
4-methyl-benzaldehyde

-
-
52287-56-6, 29816-55-5
1-(p-tolyl)-2-nitropropene

| Conditions | Yield |
|---|---|
| With ammonium acetate at 110℃; for 16h; | 100% |
| With (2-hydroxyethyl)ammonium formate at 20℃; for 4.1h; Knoevenagel condensation; Ionic liquid; | 95% |
| With ammonium acetate at 120℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| With C62H54N2O2; copper diacetate In ethanol at 10℃; for 48h; Henry Nitro Aldol Condensation; enantioselective reaction; | 100% |
| With C62H48CuN2O2; copper diacetate; benzaldehyde In ethanol at 10℃; for 48h; Reagent/catalyst; Henry Nitro Aldol Condensation; | 100% |
| With (2-hydroxyethyl)ammonium formate at 20℃; for 0.8h; Knoevenagel condensation; Ionic liquid; | 95% |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
540-63-6
ethane-1,2-dithiol

-
-
23229-29-0
2-(4-methylphenyl)-1,3-dithiolane

| Conditions | Yield |
|---|---|
| With silica gel; toluene-4-sulfonic acid In dichloromethane for 1.5h; Heating; | 100% |
| With Cu(OTf)2-SiO2 for 0.5h; Ambient temperature; | 99% |
| With PPA; silica gel In 1,2-dichloro-ethane at 20℃; for 0.5h; | 99% |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
100-63-0
phenylhydrazine

-
-
2829-25-6
4-methylbenzaldehyde phenylhydrazone

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In dichloromethane | 100% |
| In neat (no solvent) at 20℃; for 0.00277778h; | 97% |
| With oxidized single-walled carbon nanotubes(SWCNs-COOH) In ethanol at 80℃; for 1.25h; | 96% |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
74-89-5
methylamine

-
-
17972-13-3, 29086-13-3, 53699-34-6
p-methylbenzylidene-methylamine

| Conditions | Yield |
|---|---|
| at 20℃; for 12h; | 100% |
| In methanol; dichloromethane for 18h; Molecular sieve; | 86% |

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 1h; | 100% |
| for 6h; Kinetics; Molecular sieve; Reflux; | 100% |
| With magnesium sulfate In dichloromethane at 20℃; Inert atmosphere; | 65% |

| Conditions | Yield |
|---|---|
| With diisopropoxytitanium(III) tetrahydroborate In dichloromethane at -20℃; for 0.133333h; | 100% |
| With sodium tetrahydroborate In methanol at 20℃; for 1h; | 100% |
| With hydrogen at 30℃; under 22502.3 Torr; for 10h; Temperature; Autoclave; Sealed tube; Ionic liquid; chemoselective reaction; | 100% |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
5173-28-4, 5173-29-5, 24133-59-3, 66749-58-4, 66768-19-2
1,2-di-p-tolylethane-1,2-diol

| Conditions | Yield |
|---|---|
| With aluminium; potassium hydroxide In methanol at 0℃; for 1h; Inert atmosphere; | 100% |
| With (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; acetic acid In dichloromethane at 25℃; for 16h; Michael Addition; Inert atmosphere; Irradiation; | 99% |
| With naphthalene; lithium; manganese(ll) chloride In tetrahydrofuran at 22 - 26℃; for 1h; sonication; | 98% |

| Conditions | Yield |
|---|---|
| With phosphate-buffered silica gel supported KMnO4 In cyclohexane at 65℃; | 100% |
| With periodic acid; tripropylammonium fluorochromate (VI) In acetonitrile at 0℃; for 1.5h; | 100% |
| With [Cu2C6H4(CHNCH2CH2N(CH2C5H4N)2)2](2+)*2ClO4(1-)=C36H38Cu2N8(ClO4)2; oxygen In acetone at -90.16℃; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; antimony In water for 16h; | 100% |
| With titanium(III) chloride; tin(ll) chloride In water at 20℃; for 8h; Barbier reaction; | 100% |
| With tin(ll) chloride In water at 20℃; for 24h; | 99% |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
105-56-6
ethyl 2-cyanoacetate

-
-
2017-88-1
ethyl (E)-2-cyano-3-p-tolylacrylate

| Conditions | Yield |
|---|---|
| With L-proline for 0.0833333h; Knoevenagel condensation; microwave irradiation; | 100% |
| With 1,4-diaza-bicyclo[2.2.2]octane In neat liquid at 20℃; for 0.0166667h; Knoevenagel Condensation; Green chemistry; | 100% |
| With ASCPEI In ethanol at 43℃; for 3h; Knoevenagel condensation; | 99% |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
109-77-3
malononitrile

-
-
2826-25-7
2-(4-methylbenzylidene)malononitrile

| Conditions | Yield |
|---|---|
| With L-arginine at 20℃; for 0.0833333h; Knoevenagel condensation; Ionic liquid; | 100% |
| With 1,4-diaza-bicyclo[2.2.2]octane In water at 20℃; for 0.0333333h; Knoevenagel Condensation; Green chemistry; | 100% |
| With 2C9H3O6(3-)*2C27H18N6*3Ni(2+)*15H2O*5C2H6O In dichloromethane at 60℃; for 2h; Knoevenagel Condensation; Inert atmosphere; | 100% |
-
-
3528-17-4
2,3-dihydro-4H-[1]benzothiopyran-4-one

-
-
104-87-0
4-methyl-benzaldehyde

-
-
69964-53-0, 135521-92-5, 101001-08-5
(Z)-3-(4-methylbenzylidene)thiochroman-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 3h; | 100% |
| With sulfuric acid In acetic acid at 20℃; for 0.333333h; | 89% |
| With hydrogenchloride In ethanol for 0.0833333h; Ambient temperature; | 64.1% |
| With piperidine In chloroform for 5h; Reflux; | |
| With sodium hydroxide In ethanol; water at 0℃; for 0.5h; |
-
-
27353-29-3
diethyl (morpholinomethyl)phosphonate

-
-
104-87-0
4-methyl-benzaldehyde

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at 0℃; for 1.25h; | 100% |
-
-
109-80-8
1.3-propanedithiol

-
-
104-87-0
4-methyl-benzaldehyde

-
-
56637-44-6
2-(4-methylphenyl)-1,3-dithiane

| Conditions | Yield |
|---|---|
| With lithium tetrafluoroborate at 25℃; for 1h; | 100% |
| With amberlyst-15 In acetonitrile for 1h; | 99.97% |
| With nickel dichloride In dichloromethane | 99% |
-
-
563-80-4
3-methyl-butan-2-one

-
-
104-87-0
4-methyl-benzaldehyde

-
-
67962-11-2
4-methyl-1-p-tolyl-pent-1-en-3-one

| Conditions | Yield |
|---|---|
| barium dihydroxide In ethanol for 1h; Heating; | 100% |
| With sodium hydroxide In water Heating; |
-
-
35161-71-8
N-methylpropargylamine

-
-
104-87-0
4-methyl-benzaldehyde

-
-
709-86-4
N-methyl-N-(4-methylbenzyl)prop-2-yn-1-amine

| Conditions | Yield |
|---|---|
| Stage #1: 4-methyl-benzaldehyde With acetic acid In dichloromethane at 20℃; for 0.0833333h; Inert atmosphere; Stage #2: methyl(propargyl)amine In dichloromethane at 20℃; for 1h; Inert atmosphere; Stage #3: With sodium tris(acetoxy)borohydride In dichloromethane at 0℃; Inert atmosphere; | 100% |
| With hydrogenchloride; sodium cyanoborohydride 1.) methanol, 15 min, 2.) MeOH, 24 h; Multistep reaction; |
-
-
107-10-8
propylamine

-
-
104-87-0
4-methyl-benzaldehyde

-
-
99484-07-8
N-((4-methylphenyl)methylene)-1-propanamine

| Conditions | Yield |
|---|---|
| at 20℃; for 12h; | 100% |
| With 1-hydrosilatrane In neat (no solvent) at 70℃; for 23h; Sealed tube; Green chemistry; | 99% |
| In methanol at 25℃; Mechanism; Rate constant; Thermodynamic data; ΔH(excit.), ΔS(excit.); |
-
-
558-13-4
carbon tetrabromide

-
-
104-87-0
4-methyl-benzaldehyde

-
-
60512-56-3
1-(2,2-dibromovinyl)-4-methylbenzene

| Conditions | Yield |
|---|---|
| Stage #1: carbon tetrabromide With triphenylphosphine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: 4-methyl-benzaldehyde In dichloromethane at 0℃; for 2h; Inert atmosphere; | 100% |
| Stage #1: carbon tetrabromide With triphenylphosphine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: 4-methyl-benzaldehyde In dichloromethane at 0℃; for 2h; Inert atmosphere; | 100% |
| With triphenylphosphine In dichloromethane at 0℃; for 0.5h; | 98% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
104-87-0
4-methyl-benzaldehyde

-
-
66985-49-7
2-(4-methylphenyl)-2-(trimethylsilyloxy)acetonitrile

| Conditions | Yield |
|---|---|
| With Na(1+)*C7H3NO4(2-)*Cu(2+)*C7H4NO4(1-) at 20℃; for 3h; | 100% |
| Stage #1: 4-methyl-benzaldehyde With scandium tris(trifluoromethanesulfonate); 1-n-butyl-3-methylimidazolium hexafluoroantimonate at 20℃; for 0.166667h; Stage #2: trimethylsilyl cyanide at 20℃; for 0.0833333h; Inert atmosphere; | 99% |
| With C7H3O8S2(3-)*5H2O*Pr(3+) In neat (no solvent) at 40℃; for 0.5h; Inert atmosphere; | 99% |
-
-
2942-58-7
diethyl cyanophosphonate

-
-
104-87-0
4-methyl-benzaldehyde

-
-
123346-48-5
Phosphoric acid cyano-p-tolyl-methyl ester diethyl ester

| Conditions | Yield |
|---|---|
| With lithium cyanide In tetrahydrofuran Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 0℃; | 100% |
-
-
557-20-0
diethylzinc

-
-
104-87-0
4-methyl-benzaldehyde

-
-
25574-04-3, 112777-65-8, 138809-38-8, 73854-03-2
(S)-1-(4-methylphenyl)propan-1-ol

| Conditions | Yield |
|---|---|
| (1S)-1-(9-piperidylfluoren-9-yl)ethanol In hexane; toluene at 0℃; for 4h; Addition; | 100% |
| Stage #1: diethylzinc In toluene at 0℃; for 0.5h; Stage #2: 4-methyl-benzaldehyde In toluene at 0 - 20℃; for 48h; Reagent/catalyst; enantioselective reaction; | 99% |
| Stage #1: diethylzinc; 4-methyl-benzaldehyde With diphenyl-((S)-1-((S)-1-phenylethyl)aziridin-2-yl)-methanol In hexane; toluene at 0 - 25℃; for 48.5h; Inert atmosphere; Stage #2: With ammonium chloride In hexane; water; toluene Saturated solution; optical yield given as %ee; enantioselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| In diethyl ether under 7500600 Torr; for 168h; Product distribution; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| barium dihydroxide In ethanol for 1h; Heating; | 100% |
p-Tolualdehyde Specification
1. Introduction of p-Tolualdehyde
P-Tolualdehyde is a kind of clear colorless to pale yellow liquid and belongs to the classes of Aromatic Aldehydes & Derivatives (substituted); API Intermediates; Benzaldehyde (Building Blocks for Liquid Crystals); Building Blocks for Liquid Crystals; Functional Materials. Besides, it should be stored in sealed container, and put in a cool and dry place. The storage place must stay away from oxidant, the fire and heat source. also called . The IUPAC name is 4-methylbenzaldehyde.
2. Properties of p-Tolualdehyde
(1)ACD/LogP: 2.10; (2)ACD/LogD (pH 5.5): 2.1; (3)ACD/LogD (pH 7.4): 2.1; (4)ACD/BCF (pH 5.5): 23.23; (5)ACD/BCF (pH 7.4): 23.23; (6)ACD/KOC (pH 5.5): 330.69; (7)ACD/KOC (pH 7.4): 330.69; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 1; (10)Polar Surface Area: 17.07 Å2; (11)Index of Refraction: 1.557; (12)Molar Refractivity: 37.83 cm3; (13)Molar Volume: 117.3 cm3; (14)Polarizability: 14.99 ×10-24cm3; (15)Surface Tension: 37.2 dyne/cm; (16)Density: 1.023 g/cm3; (17)Flash Point: 80 °C; (18)Enthalpy of Vaporization: 44.07 kJ/mol; (19)Boiling Point: 204.5 °C at 760 mmHg; (20)Vapour Pressure: 0.263 mmHg at 25°C.
3. Structure Descriptors of p-Tolualdehyde
(1)SMILES: O=Cc1ccc(C)cc1
(2)InChI: InChI=1/C8H8O/c1-7-2-4-8(6-9)5-3-7/h2-6H,1H3
(3)InChIKey: FXLOVSHXALFLKQ-UHFFFAOYAK
4. Toxicity of p-Tolualdehyde
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 400mg/kg (400mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. OTS0533443, |
| mouse | LD50 | oral | 3200mg/kg (3200mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | National Technical Information Service. Vol. OTS0533443, |
| rat | LC | inhalation | > 2200mg/m3 (2200mg/m3) | National Technical Information Service. Vol. OTS0533443, | |
| rat | LD50 | intraperitoneal | 800mg/kg (800mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | National Technical Information Service. Vol. OTS0533443, |
| rat | LD50 | oral | 1600mg/kg (1600mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | National Technical Information Service. Vol. OTS0533443, |
| rat | LD50 | skin | 2500mg/kg (2500mg/kg) | National Technical Information Service. Vol. OTS0538243, |
5. Safety Information of p-Tolualdehyde
Hazard Symbols:
 Xn
XnRisk Codes:
R22:Harmful if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Description:
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
6. Preparation of p-Tolualdehyde
P-Tolualdehyde may be prepared from the Friedel-Crafts formylation of toluene with carbon monoxide and hydrogen chloride under Gattermann-Koch conditions:
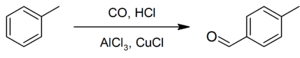
7. Use of p-Tolualdehyde
p-Tolualdehyde can be used as organic synthesis intermediates and essence. And it can react with allyl-trimethyl-silane to get 1-p-tolyl-but-3-en-1-ol. This reaction will need reagent dealuminated zeolite-Y exchanged with rare earth metal and solvent nitromethane. The reaction time is 18 hours at reaction temperature of 80 °C. The yield is about 55.0%.
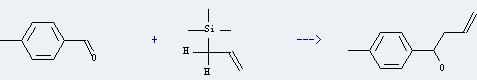
8. Other details of p-Tolualdehyde
When you are using p-Tolualdehyde, please be cautious about it as the following:
P-Tolualdehyde is harmful if swallowed and irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
Related Products
- p-Tolualdehyde
- 10487-10-2
- 10487-11-3
- 104872-06-2
- 104874-50-2
- 10487-71-5
- 104-88-1
- 104881-72-3
- 104883-49-0
- 10488-68-3
- 104-89-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View