-
Name
trans-Methyl crotonate
- EINECS 210-793-7
- CAS No. 623-43-8
- Article Data119
- CAS DataBase
- Density 0.925 g/cm3
- Solubility Immiscible with water
- Melting Point -42°C
- Formula C5H8O2
- Boiling Point 121 °C at 760 mmHg
- Molecular Weight 100.117
- Flash Point 4.4 °C
- Transport Information UN 3272 3/PG 2
- Appearance colorless Liquid
- Safety 16-26-36-9-33
- Risk Codes 11-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F, Xi
Xi
- Synonyms 2-Butenoicacid, methyl ester, (E)-;Crotonic acid, methyl ester, (E)- (8CI);(2E)-2-Butenoic acid methyl ester;(E)-2-Butenoic acid methyl ester;(E)-Crotonic acid methyl ester;Methyl (E)-2-butenoate;Methyl E-crotonate;Methyl E-propene-1-carboxylate;Methyl trans-2-butenoate;Methyltrans-crotonate;trans-2-Butenoic acid methyl ester;
- PSA 26.30000
- LogP 0.73550
Synthetic route

| Conditions | Yield |
|---|---|
| With sulfuric acid for 0.25h; Esterification; Irradiation; | 94% |
| With sulfuric acid for 12h; Heating; | 82% |
| With thionyl chloride |
-
-
67-56-1
methanol

-
-
84115-05-9
2-(diethylamino)ethyl crotonate

-
A
-
623-43-8
crotonic acid methyl ester

-
B
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 4h; Heating; | A 90% B 82% |

-
-
84115-05-9
2-(diethylamino)ethyl crotonate

-
A
-
623-43-8
crotonic acid methyl ester

-
B
-
100-37-8
2-(Diethylamino)ethanol

| Conditions | Yield |
|---|---|
| With methanol; toluene-4-sulfonic acid for 4h; Heating; | A 90% B 82% |
-
-
36056-36-7
2-ethylthio methyl ester

-
-
623-43-8
crotonic acid methyl ester

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; ammonium chloride In ethanol electrolysis; | 90% |

| Conditions | Yield |
|---|---|
| With perchloric acid; sodium percarbonate; vanadia for 1h; Heating; | 90% |
| With tert.-butylhydroperoxide In decane at 25℃; for 8h; Inert atmosphere; | 72% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel dichloride In tetrahydrofuran at 20℃; for 0.583333h; | 90% |

| Conditions | Yield |
|---|---|
| Ni(CO)4 In methanol; aqueous KCl | 90% |

-
-
124-41-4
sodium methylate

-
-
51107-31-4
2-Chloro-2-(1-chloroethyl)oxirane

-
A
-
3136-17-2, 117745-43-4, 117745-47-8
Methyl β-methoxybutyrate

-
B
-
623-43-8
crotonic acid methyl ester

-
C
-
6342-57-0
ethylglyoxal dimethylacetal

| Conditions | Yield |
|---|---|
| In methanol | A 11% B 3% C 86% |
| In methanol | A 11 % Chromat. B 3 % Chromat. C 86 % Chromat. |
| In methanol Product distribution; -50 deg C, then 3h, roomtemp.; | A 11 % Chromat. B 3 % Chromat. C 86 % Chromat. |

-
-
5469-24-9, 22426-04-6, 26708-42-9, 99570-24-8, 99570-28-2
erythro 2,3-dibromo-butenoate

-
-
623-43-8
crotonic acid methyl ester

| Conditions | Yield |
|---|---|
| With indium(III) chloride; sodium tetrahydroborate In acetonitrile at -10℃; for 3h; | 78% |
-
-
1117-71-1, 56699-18-4, 6000-00-6
methyl-4-bromo-2-butenoate

-
A
-
623-43-8
crotonic acid methyl ester

-
B
-
3724-55-8
methyl but-3-enoate

-
C
-
26474-34-0
5-Vinyl-trans-hexen-2-disaeuredimethylester

| Conditions | Yield |
|---|---|
| With zinc In dimethyl sulfoxide for 4h; Yields of byproduct given; | A n/a B n/a C 75% |


| Conditions | Yield |
|---|---|
| sulfuric acid In methanol for 2h; Heating; | 73% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 3.5h; Heating; | 72% |

| Conditions | Yield |
|---|---|
| for 2h; Solid phase reaction; esterification; | 66% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0) In N,N-dimethyl-formamide at 0℃; for 1h; | A 55% B n/a |

-
-
124-41-4
sodium methylate

-
-
16714-77-5
1,3-dichlorobutan-2-one

-
A
-
3136-17-2, 117745-43-4, 117745-47-8
Methyl β-methoxybutyrate

-
B
-
4358-59-2
(Z)-methyl but-2-enoate

-
C
-
623-43-8
crotonic acid methyl ester

-
D
-
6342-57-0
ethylglyoxal dimethylacetal

| Conditions | Yield |
|---|---|
| In methanol -50 deg C, then 3h, roomtemp.; Further byproducts given; | A 13% B 36% C 16% D 16% |

-
-
124-41-4
sodium methylate

-
-
16714-77-5
1,3-dichlorobutan-2-one

-
A
-
3136-17-2, 117745-43-4, 117745-47-8
Methyl β-methoxybutyrate

-
B
-
4358-59-2
(Z)-methyl but-2-enoate

-
C
-
623-43-8
crotonic acid methyl ester

-
D
-
6342-57-0
ethylglyoxal dimethylacetal

-
E
-
80-62-6
methacrylic acid methyl ester

| Conditions | Yield |
|---|---|
| In methanol Product distribution; -50 deg C, then 3h, roomtemp.; | A 13% B 36% C 16% D 16% E 13% |

-
-
124-41-4
sodium methylate

-
-
16714-77-5
1,3-dichlorobutan-2-one

-
A
-
4358-59-2
(Z)-methyl but-2-enoate

-
B
-
623-43-8
crotonic acid methyl ester

-
C
-
6342-57-0
ethylglyoxal dimethylacetal

-
D
-
80-62-6
methacrylic acid methyl ester

| Conditions | Yield |
|---|---|
| In methanol -50 deg C, then 3h, roomtemp.; Further byproducts given; | A 36% B 16% C 16% D 13% |

-
-
35466-83-2
allyl methyl carbonate

-
-
201230-82-2
carbon monoxide

-
A
-
623-43-8
crotonic acid methyl ester

-
B
-
3724-55-8
methyl but-3-enoate

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; dodecacarbonyl-triangulo-triruthenium at 120℃; under 30400 Torr; for 10h; Yields of byproduct given; | A 18% B n/a |


| Conditions | Yield |
|---|---|
| 4% |

| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With hydrogenchloride | |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With mineral acid |
-
-
922-69-0
1,1-dimethoxyethylene

-
-
75-07-0
acetaldehyde

-
A
-
1445-45-0
Trimethyl orthoacetate

-
B
-
123-73-9
trans-Crotonaldehyde

-
C
-
623-43-8
crotonic acid methyl ester

-
D
-
79-20-9
acetic acid methyl ester

| Conditions | Yield |
|---|---|
| at 150℃; |

-
-
3068-88-0, 32082-74-9, 36536-46-6, 65058-82-4
4-methyloxetan-2-one

-
-
74-88-4
methyl iodide

-
-
623-43-8
crotonic acid methyl ester

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium naphthalenide 1) THF, 20 deg C, 10 min; Yield given. Multistep reaction; |
-
-
67-56-1
methanol

-
-
625-35-4, 3488-22-0, 10487-71-5
trans-chrotonyl chloride

-
A
-
623-43-8
crotonic acid methyl ester

-
B
-
3724-55-8
methyl but-3-enoate

| Conditions | Yield |
|---|---|
| With 2,4-dimethylaminopyridine; N-ethyl-N,N-diisopropylamine In dichloromethane for 0.5h; Yield given. Yields of byproduct given; |

-
-
67-56-1
methanol

-
-
15081-72-8
4,4-bis-methylsulfanyl-but-3-en-2-ol

-
-
623-43-8
crotonic acid methyl ester

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate 1) room temp., 5 min, 2) reflux, 16 h; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 9h; Heating; | 1.28 g |
-
-
108-55-4
glutaric anhydride,

-
A
-
542-28-9
3,4,5,6-tetrahydro-2H-pyran-2-one

-
B
-
623-43-8
crotonic acid methyl ester

-
C
-
623-42-7
butanoic acid methyl ester

-
D
-
3724-55-8
methyl but-3-enoate

-
E
-
106-73-0
methyl heptanoate

-
F
-
1732-09-8
dimethyl subarate

| Conditions | Yield |
|---|---|
| With electrochemical reduction Product distribution; by-products; |

-
-
75-52-5
nitromethane

-
-
623-43-8
crotonic acid methyl ester

-
-
16507-06-5
methyl 3-methyl-4-nitro-butyrate

| Conditions | Yield |
|---|---|
| With N,N,N',N'-tetramethylguanidine at 20℃; for 576h; | 100% |
| With DBN In methanol at 60℃; for 6h; Inert atmosphere; | 71.44% |
| With methanol; potassium carbonate | |
| With N-benzyl-trimethylammonium hydroxide In methanol | |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In methanol Michael addition; |

| Conditions | Yield |
|---|---|
| With bromine In dichloromethane at 0 - 20℃; Inert atmosphere; | 100% |
| With bromine In acetic acid at 30℃; Mechanism; Rate constant; | |
| With bromine | |
| With bromine In tetrachloromethane at 0℃; for 0.5h; | |
| With bromine In n-heptane at 25 - 35℃; for 1.33333 - 1.5h; |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; methylcopper; diisobutylaluminium hydride In tetrahydrofuran; diethyl ether; hexane at -50℃; for 0.5h; | 100% |
| With hydrogen; Rhodium chloride tri(triphenylphosphine-meta-trisulfonate) In water for 16h; Ambient temperature; | 95% |
| With hydrogen; Pd(II) complex containing tridentate hydrazonic ligands In methanol at 40℃; for 24h; Hydrogenation; | 35% |
| With hydrogen; montmorillonite-(bipyridine)x In benzene at 75℃; under 31028.9 Torr; for 45h; | 91 % Spectr. |
| With hydrogen In methanol at 80℃; under 37503.8 Torr; for 20h; Autoclave; |
-
-
110-89-4
piperidine

-
-
623-43-8
crotonic acid methyl ester

-
-
122958-11-6
(+/-)-3-(1-piperidinyl)butyric acid methyl ester

| Conditions | Yield |
|---|---|
| [(dppf)Pd(NCMe)(H2O)](OTf)2 In toluene at 20℃; for 18h; | 100% |
| In methanol at 20℃; for 168h; Addition; | 85% |
| With 3-butyl-1-methylimidazolium hydroxide at 25℃; for 1.5h; aza-Michael addition; | 85% |

| Conditions | Yield |
|---|---|
| With magnesium oxide In toluene at 20℃; for 0.5h; Michael addition; | 100% |
-
-
623-43-8
crotonic acid methyl ester

-
-
10234-76-1
5-methyl-pyrazolidin-3-one

| Conditions | Yield |
|---|---|
| With hydrazine hydrate | 100% |
-
-
623-43-8
crotonic acid methyl ester

-
-
103-67-3
benzyl-methyl-amine

-
-
40871-09-8
methyl 3-(N-benzyl-N-methylamino)-3-methylpropionate

| Conditions | Yield |
|---|---|
| 100% | |
| 100% | |
| 100% | |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 50℃; for 14h; aza-Michael addition; | 77% |
-
-
623-43-8
crotonic acid methyl ester

-
-
112-12-9
methyl nonyl ketone

-
-
111748-84-6, 111748-88-0
4,5-Dimethyl-5-nonyl-dihydro-furan-2-one

| Conditions | Yield |
|---|---|
| With samarium diiodide; isopropyl alcohol In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide at 0℃; for 0.0166667h; | 99% |
-
-
623-43-8
crotonic acid methyl ester

-
-
33356-45-5
2-(Trimethylsilyl)benzenethiol

| Conditions | Yield |
|---|---|
| With 2-TMSC6H4SLi; (-)-(1R,2R)-1-dimethylamino-2-(2-methoxyphenoxy)-1,2-diphenylethane In hexane; toluene at -78℃; for 120h; Addition; | 99% |
-
-
623-43-8
crotonic acid methyl ester

-
-
100-46-9
benzylamine

-
-
507444-65-7
methyl 3-(benzylamino)butanoate

| Conditions | Yield |
|---|---|
| In ethanol for 16h; Heating; | 99% |
| In methanol at 150℃; under 5688.78 Torr; for 3h; Michael addition; microwave irradiation; | 98% |
| In methanol at 20℃; for 2h; | 96% |
-
-
623-43-8
crotonic acid methyl ester

-
-
81477-94-3
N-(diphenylmethylene)glycine tert-butyl ester

| Conditions | Yield |
|---|---|
| With 4 A molecular sieve; calcium bis-iso-propoxide; 2,2'-methylene bis((4S)-4-phenyl-2-oxazoline) In tetrahydrofuran at -30℃; for 12h; Michael addition; | 99% |

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole In dimethyl sulfoxide at 70℃; for 12h; Michael addition; | 99% |
| With acylase Amano from Aspergillus oryzae In dimethyl sulfoxide at 50℃; for 24h; Michael addition reaction; | 37% |

| Conditions | Yield |
|---|---|
| With C49H53N4O4PS In toluene at 20℃; for 3h; Reagent/catalyst; enantioselective reaction; | A n/a B 99% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80℃; for 24h; Michael Addition; Schlenk technique; Sealed tube; | 98.5% |

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide; isopropyl alcohol for 0.0166667h; | 98% |
-
-
623-43-8
crotonic acid methyl ester

-
-
124-46-9, 20734-13-8, 100224-74-6, 593-85-1
guanidine hydrogen carbonate

-
-
109-77-3
malononitrile

-
-
97934-10-6
2,4-diamino-5,6-dihydro-5-methylpyrido<2,3-d>pyrimidin-7(8H)-one

| Conditions | Yield |
|---|---|
| Stage #1: guanidine hydrogen carbonate With sodium methylate In methanol for 0.25h; Heating; Stage #2: crotonic acid methyl ester; malononitrile In methanol at 140℃; for 0.166667h; Irradiation; | 98% |

-
-
623-43-8
crotonic acid methyl ester

-
-
124-46-9, 20734-13-8, 100224-74-6, 593-85-1
guanidine hydrogen carbonate

-
-
105-34-0
methyl 2-cyanoacetate

| Conditions | Yield |
|---|---|
| Stage #1: guanidine hydrogen carbonate With sodium methylate In methanol for 0.25h; Heating; Stage #2: crotonic acid methyl ester; methyl 2-cyanoacetate In methanol at 140℃; for 0.166667h; Irradiation; | 98% |

-
-
930122-16-0
(6-oxo-4-oxy-3,6-dihydro-2H-pyrazin-1-yl)-acetic acid methyl ester

-
-
623-43-8
crotonic acid methyl ester

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate In tetrahydrofuran for 14h; Heating; | 98% |
-
-
70957-05-0
1-(N,N‑dimethylcarbamoyl)indole

-
-
623-43-8
crotonic acid methyl ester

-
-
1454704-66-5
(E)-methyl 3-(1-(dimethylcarbamoyl)-1H-indol-2-yl)but-2-enoate

| Conditions | Yield |
|---|---|
| With tris(acetonitrile)(η5-pentamethylcyclopentadienyl)rhodium(III) hexafluoroantimonate; copper(II) acetate monohydrate In tetrahydrofuran at 100℃; for 24h; Heck Reaction; Inert atmosphere; stereoselective reaction; | 98% |
| With silver hexafluoroantimonate; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2; copper(II) acetate monohydrate In 1,4-dioxane at 100℃; for 30h; Schlenk technique; Inert atmosphere; regioselective reaction; | 90% |

-
-
623-43-8
crotonic acid methyl ester

| Conditions | Yield |
|---|---|
| With C34H43N4O6Zn(1+)*CF3O3S(1-) In acetonitrile at 50℃; for 24h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 98% |
-
-
288-32-4
1H-imidazole

-
-
623-43-8
crotonic acid methyl ester

-
-
1153450-51-1
3-imidazol-1-yl-butyric acid methyl ester

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 80℃; for 24h; Michael Addition; Schlenk technique; Sealed tube; | 97.5% |
-
-
623-43-8
crotonic acid methyl ester

-
-
100-52-7
benzaldehyde

-
-
72853-47-5
5-hydroxy-5-phenyl-pent-2-enoic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: crotonic acid methyl ester; benzaldehyde With aluminium tris(2,6-diphenylphenoxide) In toluene at -78℃; for 0.333333h; Complexation; Stage #2: With 2,2,6,6-tetramethylpiperidinyl-lithium In tetrahydrofuran; hexane; toluene at -78℃; for 0.5h; Crossed aldol condensation; | 97% |
| Stage #1: crotonic acid methyl ester; benzaldehyde With aluminium tris(2,6-diphenylphenoxide) In toluene at -78℃; for 0.0833333h; Stage #2: With 2,2,6,6-tetramethylpiperidinyl-lithium In tetrahydrofuran; hexane; toluene at -78℃; for 0.5h; | 97% |
| Stage #1: crotonic acid methyl ester; benzaldehyde With aluminum tris(2,6-di-2-naphthylphenoxide) In toluene at -78℃; for 0.333333h; Inert atmosphere; Stage #2: With 2,2,6,6-tetramethylpiperidinyl-lithium In tetrahydrofuran; toluene at -78℃; for 3h; Inert atmosphere; Stage #3: With ammonium chloride In tetrahydrofuran; toluene at -78 - 20℃; for 0.5h; Inert atmosphere; | 97% |
-
-
623-43-8
crotonic acid methyl ester

| Conditions | Yield |
|---|---|
| With CF3O3S(1-)*C32H40N4O4*Zn(2+)*C2H3O2(1-) In acetonitrile at 80℃; for 60h; enantioselective reaction; | 97% |
-
-
623-43-8
crotonic acid methyl ester

-
-
108-59-8
malonic acid dimethyl ester

-
-
65844-74-8
2-methylpropan-1,1,3-tricarboxylic acid trimethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: malonic acid dimethyl ester With sodium methylate In tetrahydrofuran; methanol at 23℃; for 0.166667h; Stage #2: crotonic acid methyl ester In tetrahydrofuran; methanol at 45 - 50℃; for 4.16667h; | 96% |
| With sodium methylate In methanol Heating; | 84% |
-
-
623-43-8
crotonic acid methyl ester

-
-
2043-61-0
cyclohexanecarbaldehyde

-
-
111748-83-5, 111748-87-9, 133101-54-9, 133101-64-1
cis-(RS,SR)-γ-cyclohexyl-γ-hydroxy-β-methylbutanoic acid lactone

| Conditions | Yield |
|---|---|
| With samarium diiodide; isopropyl alcohol In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide at 0℃; for 0.0166667h; Product distribution; without HMPA, further reaction time; | 96% |
-
-
623-43-8
crotonic acid methyl ester

-
-
22038-57-9, 60753-84-6, 22038-58-0
(Z) methyl 2-chloro-2-butenoate

| Conditions | Yield |
|---|---|
| With aluminium trichloride; benzeneseleninyl chloride In dichloromethane at 40℃; for 23h; | 96% |
| With chlorine In dichloromethane for 0.666667h; | 34% |
| Multi-step reaction with 2 steps 1: Cl2 / CCl4 / 48 h / 0 °C 2: triethylamine / 72 h View Scheme | |
| Multi-step reaction with 2 steps 1: Cl2, Br2 / CCl4 / 48 h / 0 °C 2: triethylamine / 72 h View Scheme |
-
-
623-43-8
crotonic acid methyl ester

| Conditions | Yield |
|---|---|
| With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 1h; | 96% |
-
-
623-43-8
crotonic acid methyl ester

-
-
182316-01-4
(5R)-5,6-dihydro-5-phenyl-2H-1,4-oxazin-2-one-N-oxide

| Conditions | Yield |
|---|---|
| In chloroform for 3h; Ambient temperature; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: crotonic acid methyl ester; cyclohexanecarbaldehyde With aluminum tris(2,6-di-2-naphthylphenoxide) In toluene at -78℃; for 0.333333h; Inert atmosphere; Stage #2: With 2,2,6,6-tetramethylpiperidinyl-lithium In tetrahydrofuran; toluene at -78℃; for 3h; Inert atmosphere; Stage #3: With ammonium chloride In tetrahydrofuran; toluene at -78 - 20℃; for 0.5h; Inert atmosphere; | 96% |
| Stage #1: crotonic acid methyl ester; cyclohexanecarbaldehyde With aluminium tris(2,6-diphenylphenoxide) In toluene at -78℃; for 0.333333h; Complexation; Stage #2: With 2,2,6,6-tetramethylpiperidinyl-lithium In tetrahydrofuran; hexane; toluene at -78℃; for 0.5h; Crossed aldol condensation; | 90% |

| Conditions | Yield |
|---|---|
| With 3-methylpyridin-2-ylamine; benzoic acid; Wilkinson's catalyst In toluene at 150℃; for 72h; | 96% |
trans-Methyl crotonate Chemical Properties
Molecular Formula: C5H8O2
Formula Weight: 100.13g/mol
Appearance: colorless liquid
Density: 0.944g/cm3
Boiling Point: 121 °C at 760 mmHg
Rrefractive index: n20/D 1.423(lit.)
Flash Point: 4.4 °C
Vapor Pressure: 14.9 mmHg at 25°C
Solubility: dissolved in alcohol and ether,not soluble in water
EINECS: 210-793-7
liansport Information: UN 3272
Freely Rotating Bonds: 2
Index of Refraction: 1.414
Molar Refractivity: 27.04 cm3
Molar Volume: 108.1 cm3
Polarizability: 10.72 10-24cm3
Surface Tension: 25.4 dyne/cm
Enthalpy of Vaporization: 35.91 kJ/mol
The chemical synonyms of (E)-CROTONIC ACID METHYL ESTER(623-43-8) are (E)-2-Butenoic acid methyl ester;(E)-But-2-enoicacidmethylester;(E)-CH3CH=CHC(O)OCH3;(E)-Crotonic acid methyl ester;Crotonic acid, methyl ester, (E)-;Methyl (2E)-2-butenoate;methyl (E)-2-butenoate;Methyl 2-butenoate, (E)- .Its product categories is C2 to C5;Carbonyl Compounds and Esters.The molecular structure of (E)-CROTONIC ACID METHYL ESTER(623-43-8) is
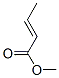 .
.trans-Methyl crotonate Uses
trans-Methyl crotonate Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MOD | FCTXAV Food and Cosmetics Toxicology. 17 (1979),865. | ||
| 2. | orl-mus LD50:1600 mg/kg | FCTXAV Food and Cosmetics Toxicology. 17 (1979),865. | ||
| 3. | skn-gpg LD50:10 g/kg | FCTXAV Food and Cosmetics Toxicology. 17 (1979),865. |
trans-Methyl crotonate Consensus Reports
trans-Methyl crotonate Safety Profile
Hazard Symbols:
 F;
F; Xi
XiF: Highly flammable
Xi Irritant
Risk Statements 11-36/37/38
11: Highly flammable.
36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements 16-26-36-9-33
16: Keep away from sources of ignition - No smoking.
33: Take precautionary measures against static discharges.
9: Keep container in a well-ventilated place. 36: Wear suitable protective clothing
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
HazardClass: 3
trans-Methyl crotonate Specification
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View