-
Name
1,4-Dimethylbenzene
- EINECS 203-396-5
- CAS No. 106-42-3
- Article Data698
- CAS DataBase
- Density 0.87 g/cm3
- Solubility miscible with ethanol, ether, benzene and acetone, insoluble in water
- Melting Point 12-13 °C(lit.)
- Formula C8H10
- Boiling Point 139.61 °C at 760 mmHg
- Molecular Weight 106.167
- Flash Point 27.22 °C
- Transport Information UN 1307 3/PG 3
- Appearance colourless liquid
- Safety 25-45-36/37
- Risk Codes 10-20/21-38-39/23/24/25-23/24/25
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, T
T
- Synonyms p-Xylene(8CI);1,4-Xylene;4-Methyltoluene;NSC 72419;p-Dimethylbenzene;p-Methyltoluene;p-Phenylenebis(methylene);p-Xylol;1,4-Dimethylbenzene;
- PSA 0.00000
- LogP 2.30340
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen In water; ethyl acetate at 50℃; under 15001.5 Torr; for 5h; | 99% |
| With hydrogen In water at 25℃; for 1h; | 99% |
| With hydrogen at 350℃; under 760.051 Torr; | 95% |

| Conditions | Yield |
|---|---|
| With solid acid catalyst tin phosphate In n-heptane at 300℃; under 15001.5 Torr; for 12h; Reagent/catalyst; Solvent; Pressure; Temperature; | 90% |
| With copper(II) bis(trifluoromethanesulfonate) at 250 - 270℃; under 26892.4 - 82745.9 Torr; for 5h; Inert atmosphere; | 44% |

| Conditions | Yield |
|---|---|
| With P-containing zeolite Beta In n-heptane at 250℃; under 46504.7 Torr; Reagent/catalyst; Diels-Alder Cycloaddition; | 97% |
| With benzoic acid anhydride In acetic acid at 280℃; under 63006.3 Torr; for 8h; Pressure; Reagent/catalyst; Solvent; Temperature; Time; | 92.3% |
| With copper(II) bis(trifluoromethanesulfonate) In tetrahydrofuran at 270℃; under 26892.4 - 82745.9 Torr; for 5h; Catalytic behavior; Reagent/catalyst; Solvent; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| In n-heptane at 249.84℃; under 37503.8 Torr; for 24h; Reagent/catalyst; Autoclave; Inert atmosphere; | A 75% B n/a |
| In acetic acid at 280℃; under 63006.3 Torr; for 4h; Pressure; Reagent/catalyst; Solvent; Temperature; Time; | A 68.5% B 12.1% |
| With Sn-BEA In n-heptane at 250℃; under 46504.7 Torr; for 24h; Catalytic behavior; Kinetics; Diels-Alder Cycloaddition; Autoclave; Inert atmosphere; chemoselective reaction; | A 43% B n/a |


| Conditions | Yield |
|---|---|
| With hydrogen; P-modified ZSM-5 In water at 480 - 485℃; under 1794.37 Torr; for 23.53h; Product distribution / selectivity; | A n/a B 15.54% C n/a |
| H-ZSM-5(73) at 400℃; Product distribution; further catalysts (alumosilicates, modified zeolites); | |
| Na(+)x(Mg3)(Si(4-x)Alx)O10(OH)2 at 349.9℃; for 1h; Product distribution; various pillared clays and zeolites as catalysts; |


| Conditions | Yield |
|---|---|
| With hydrogen In water at 400℃; under 3750.38 Torr; Reagent/catalyst; Pressure; Temperature; Concentration; Inert atmosphere; | 29.3% |
| With hydrogen at 250℃; Kinetics; Thermodynamic data; Further Variations:; pH-values; | |
| silica bound HZSM-5 zeolite at 600℃; under 3620.13 - 4137.29 Torr; for 3.25 - 7.5h; Conversion of starting material; |
-
-
589-18-4
4-Methylbenzyl alcohol

-
-
55-21-0
benzamide

-
A
-
106-42-3
para-xylene

-
B
-
65608-94-8
N-(p-methylbenzyl)benzamide

| Conditions | Yield |
|---|---|
| With barium trifluoromethanesulfonate In toluene at 150℃; for 18h; Reagent/catalyst; Glovebox; Inert atmosphere; | A 29% B 72% |


| Conditions | Yield |
|---|---|
| With hydrogen; silicate ITQ-13, aluminium form at 360℃; under 10343.2 Torr; Product distribution / selectivity; fixed-bed downflow reactor; | 23.5% |
| MTW-zeolite/Al2O3 (Catalyst B) at 280℃; under 26618.1 Torr; Conversion of starting material; Liquid phase; | |
| Ga-MFI zeolite/ZrO2/Al2O3 (Catalyst C) at 300℃; under 26618.1 Torr; Conversion of starting material; Liquid phase; |
-
-
625-86-5
2,5-dimethylfuran

-
-
74-85-1
ethene

-
A
-
106-42-3
para-xylene

-
B
-
15329-10-9
3,6-dimethylcyclohex-2-enone

-
C
-
110-13-4
2,5-hexanedione

| Conditions | Yield |
|---|---|
| With H-BEA-25 In 1,4-dioxane at 249.84℃; under 15001.5 Torr; for 4h; Diels-Alder Cycloaddition; Autoclave; chemoselective reaction; |


| Conditions | Yield |
|---|---|
| With palladium on activated charcoal In toluene at 130℃; under 7500.75 - 30003 Torr; for 3h; Autoclave; |
-
-
111957-97-2
1,4-dimethyl-7-oxa-bicyclo[2.2.1]heptane-2,3-dicarboxylic acid anhydride

-
A
-
5463-50-3
3,6-dimethyl phthalic anhydride

-
B
-
106-42-3
para-xylene

| Conditions | Yield |
|---|---|
| With zeolite Y with a silica-alumina at 200℃; for 2h; Temperature; Inert atmosphere; | A 72% B 17% |
| With 1 wtpercent Pd/C loaded on zeolite H-Y with a silica-alumina ratio of 40 In toluene at 200℃; under 750.075 Torr; for 4h; Inert atmosphere; |
-
-
589-18-4
4-Methylbenzyl alcohol

-
-
55-21-0
benzamide

-
A
-
106-42-3
para-xylene

-
B
-
65608-94-8
N-(p-methylbenzyl)benzamide

-
C
-
40891-10-9
N,N′‑(4‑methylbenzylidene)bisbenzamide

| Conditions | Yield |
|---|---|
| With indium sulfate In toluene at 150℃; for 18h; Glovebox; Inert atmosphere; | A 60% B 14% C 40% |
| With zinc(II) sulfate In toluene at 150℃; for 18h; Glovebox; Inert atmosphere; | A 41% B 58% C 6% |
| With zinc(II) chloride In toluene at 150℃; for 18h; Glovebox; Inert atmosphere; | A 47% B 42% C 21% |


| Conditions | Yield |
|---|---|
| With hydrogen at 480℃; under 8826.09 Torr; Leiten ueber Platin/Aluminiumoxid-Siliciumdioxid; | |
| With hydrogen; 0.3 wtpercent Pt/0.1 wtpercent S/100 wtpercent MTW-zeolite (Catatalyst C) at 370 - 375℃; under 4650.47 Torr; Conversion of starting material; | |
| With hydrogen; 0.3 wtpercent Pt/0.1 wtpercent S/50 wtpercent MTW/50 wtpercent mordenite (Catalyst F) at 370 - 375℃; under 4650.47 Torr; Conversion of starting material; |
-
-
67-56-1
methanol

-
-
108-88-3
toluene

-
-
71-43-2
benzene

-
A
-
95-47-6
o-xylene

-
B
-
106-42-3
para-xylene

-
C
-
108-38-3
m-xylene

| Conditions | Yield |
|---|---|
| With hydrogen; 1/16 inch extrudates which contained 65 weight percent H-ZSM-5 and 35 weight percent silica binder at 371.101℃; under 3309.83 Torr; | |
| With hydrogen; 1/16 inch extrudates which contained 65 wt. percent H-ZSM-23 having a silica to alumina mole ratio of 110:1 and 35 wt. percent of alumina binder at 499.99℃; under 7757.43 Torr; | |
| With hydrogen; SAPO-11 at 499.99℃; under 7757.43 Torr; |


| Conditions | Yield |
|---|---|
| With hydrogen; silica selectivated ZSM-5 at 371.101℃; under 10343.2 Torr; |


| Conditions | Yield |
|---|---|
| With triethylsilane; palladium dichloride In ethanol for 0.5h; Inert atmosphere; | A 10.0 %Chromat. B 88.5 %Chromat. |
| With palladium on silica; hydrogen In dodecane at 190℃; under 22502.3 Torr; for 5h; | |
| With palladium on activated charcoal; hydrogen In water at 100℃; for 10h; chemoselective reaction; | A 40 %Chromat. B 60 %Chromat. |

| Conditions | Yield |
|---|---|
| With parent microporous ZSM-5 zeolite at 250℃; for 0.25h; Reagent/catalyst; | A 27% B 10% |
| With hydrogen; H-ZSM-5 at 349.9℃; under 15001.2 Torr; Product distribution; further catalysts, variation of temperature; | |
| CVD boria-alumina at 450℃; Product distribution; dependence of conversion on B2O3 content of catalyst; |

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; {MoPdcp[μ-(CO)2][μ3-(CO)](PPh3)}2 In benzene at 120℃; for 15h; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogen; platinum-containing ZSM-5 molecular sieve catalyst at 420℃; under 11251.1 Torr; | |
| With hydrogen; alumina-phosphate-bound MFI catalyst at 560℃; under 18376.8 Torr; Gas phase; | |
| With hydrogen; alumina-phosphate-bound MFI catalyst at 560℃; under 18376.8 Torr; Gas phase; |
-
-
589-18-4
4-Methylbenzyl alcohol

-
-
55-21-0
benzamide

-
A
-
106-42-3
para-xylene

-
B
-
65608-94-8
N-(p-methylbenzyl)benzamide

-
C
-
38460-98-9
bis(4-methylbenzyl) ether

| Conditions | Yield |
|---|---|
| With tin(II) trifluoromethanesulfonate In toluene at 150℃; for 18h; Glovebox; Inert atmosphere; | A 8% B 33% C 50% |
| With zinc trifluoromethanesulfonate In toluene at 150℃; for 18h; Reagent/catalyst; Glovebox; Inert atmosphere; | A 33% B 22% C 21% |

-
-
625-86-5
2,5-dimethylfuran

-
-
74-85-1
ethene

-
A
-
106-42-3
para-xylene

-
B
-
1074-55-1
p-n-propyltoluene

-
C
-
1758-88-9
2-ethyl-p-xylene

| Conditions | Yield |
|---|---|
| With palladium-decorated gold nanoparticles anchored on amphoteric zirconia In n-heptane at 300℃; under 30402 Torr; for 6h; |

-
-
625-86-5
2,5-dimethylfuran

-
-
74-85-1
ethene

-
A
-
1073-13-8
4,4-dimethylcyclohexenone

-
B
-
106-42-3
para-xylene

-
C
-
15329-10-9
3,6-dimethylcyclohex-2-enone

| Conditions | Yield |
|---|---|
| With Sn-BEA In n-heptane at 275℃; under 30402 Torr; for 1h; |


| Conditions | Yield |
|---|---|
| With palladium/alumina; hydrogen In hexane at 325℃; Flow reactor; Green chemistry; | A 84.8% B 6.5% |
| With hydrogen In hexane at 325℃; Flow reactor; Green chemistry; | A 19.5% B 73.2% |

| Conditions | Yield |
|---|---|
| With copper nickel; pyrographite In 1,2-dimethoxyethane at 85℃; for 20h; | A 10% B 90% |
| With nickel In 1,2-dimethoxyethane for 2h; Ambient temperature; | A 20% B 76% |
| With magnesium at 600℃; | A 23% B 60% |
| With water; naphthalen-1-yl-lithium In tetrahydrofuran; diethyl ether; Petroleum ether at -95℃; for 0.75h; Title compound not separated from byproducts; | A 64 % Chromat. B 36 % Chromat. |

| Conditions | Yield |
|---|---|
| With Ga2O3 zeolite; silica gel In gas at 400℃; Product distribution; var. catalyst, var. temp.; | |
| With hydrogen; Catalyst G (prepared from NH4-ZSM-5 and H3PO4) at 200 - 500℃; under 1034.32 Torr; Product distribution / selectivity; | |
| With hydrogen; Catalyst H (prepared from NH4-ZSM-5 and H3PO4) at 200 - 500℃; under 1034.32 Torr; Product distribution / selectivity; |

-
-
201230-82-2
carbon monoxide

-
-
589-29-7
p-xylylene glycol

-
A
-
106-42-3
para-xylene

-
B
-
7325-46-4
1,4-phenylenediacetic acid

-
C
-
622-47-9
4-tolylacetic acid

| Conditions | Yield |
|---|---|
| With hydrogen iodide; tetrakis(triphenylphosphine) palladium(0) In acetone at 90℃; under 68400 Torr; for 42h; Carbonylation; reduction; | A n/a B 48% C 16% |

-
-
67-56-1
methanol

-
-
108-88-3
toluene

-
-
71-43-2
benzene

-
A
-
95-47-6
o-xylene

-
B
-
106-42-3
para-xylene

-
C
-
100-41-4
ethylbenzene

-
D
-
108-38-3
m-xylene

| Conditions | Yield |
|---|---|
| With hydrogen; silicon-selectivated H-ZSM-5/silica bound at 371.101℃; under 10343.2 Torr; | |
| With hydrogen; zeolite bound zeolite at 371.101℃; under 10343.2 Torr; |


| Conditions | Yield |
|---|---|
| catalyst contained 15.0percentw EU-1 zeolite in H form, 84.7percentw Al2O3, 0.3percentw Pt and treated with dimethyldisulphide at 390℃; under 11251.1 Torr; Product distribution / selectivity; | |
| catalyst contained 15.0percentw NU-87 zeolite in H form, 84.7percentw Al2O3, 0.3percentw Pt and treated with dimethyldisulphide at 390℃; under 11251.1 Torr; Product distribution / selectivity; | |
| catalyst contained 8.0percentw EU-1 zeolite in H form, 7.0percentw NU-87 zeolite in H form, 84.7percentw Al2O3, 0.15percentw Pt, 0.15percentw Re and treated with dimethyldisulphide at 390℃; under 11251.1 Torr; Product distribution / selectivity; |


| Conditions | Yield |
|---|---|
| With palladium dichloride In methanol at 40℃; for 18h; Inert atmosphere; Green chemistry; chemoselective reaction; | 99% |
| Multi-step reaction with 2 steps 1: hydrogen; / 350 °C / 760.05 Torr 2: hydrogen; / 350 °C / 760.05 Torr View Scheme | |
| With formic acid; methanesulfonic acid; 1,2-bis((di-tert-butylphosphoryl)methyl)benzene; palladium(II) acetylacetonate; 1,2-bis[di(t-butyl)phosphinomethyl]benzene In 1,2-dichloro-ethane at 100℃; for 18h; Schlenk technique; Sealed tube; Inert atmosphere; | 93 %Chromat. |
| With 2,4,6-trimethyl-pyridine; 4,4'-dimethoxyphenyl disulfide; iridium(lll) bis[2-(2,4-difluorophenyl)-5-methylpyridine-N,C20]-4,40-di-tert-butyl-2,20-bipyridine hexafluorophosphate; triphenylphosphine In toluene for 24h; Irradiation; | 63 %Chromat. |
-
-
201230-82-2
carbon monoxide

-
-
108-88-3
toluene

-
A
-
95-47-6
o-xylene

-
B
-
106-42-3
para-xylene

-
C
-
108-38-3
m-xylene

| Conditions | Yield |
|---|---|
| With hydrogen at 400℃; under 24549.5 Torr; Temperature; Flow reactor; Inert atmosphere; |


| Conditions | Yield |
|---|---|
| With potassium bromide; sodium nitrite In water; trifluoroacetic acid at 20℃; for 0.16h; Product distribution; under argon; | 100% |
| With oxygen; potassium bromide; sodium nitrite In water; trifluoroacetic acid at 20℃; for 5h; Product distribution; | 96% |
| With sulfuric acid; dihydrogen peroxide; sodium bromide In water at 49.84℃; | 94% |

| Conditions | Yield |
|---|---|
| With zeolite ZSM-5-60; Nitrogen dioxide | 100% |
| With nitric acid; sulfuric acid In dichloromethane at 25℃; for 0.7h; | 100% |
| With nitric acid In toluene at 60℃; for 2h; | 98% |

| Conditions | Yield |
|---|---|
| With oxygen; 10-methyl-9-phenylacridin-10-ium perchlorate In chloroform at 24.84℃; for 10h; Oxidation; Pyrolysis; visible light; | 100% |
| With sulfuric acid; 9-mesityl-2,7,10-trimethylacridinium perchlorate; water; oxygen In acetonitrile at 24.84℃; for 1.33333h; Quantum yield; Reagent/catalyst; Irradiation; | 100% |
| With N-hydroxyphthalimide; oxygen; cobalt(II) acetate; acetic acid at 20℃; under 760.051 Torr; chemoselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With bromine In tetrachloromethane Solvent; | 100% |
| With 2,4,4,6-Tetrabromo-2,5-cyclohexadien-1-one; silica gel for 4.5h; UV-irradiation; | 84% |
| With bromine In tetrachloromethane for 1.5h; Ambient temperature; | 83% |

| Conditions | Yield |
|---|---|
| With hydrogen; [(norbornadiene)rhodium(I)chloride]2; phosphinated polydiacetylene In n-heptane at 30℃; under 60800 Torr; for 1.5h; | 100% |
| With Ti8O8(14+)*6C8H4O4(2-)*4O(2-)*3.3Li(1+)*0.7Co(2+)*0.7C4H8O*0.7H(1-); hydrogen In neat (no solvent) at 120℃; under 37503.8 Torr; for 18h; | 100% |
| With hydrogen at 150℃; under 2280.15 Torr; for 0.5h; Kinetics; Reagent/catalyst; Temperature; | 100% |

| Conditions | Yield |
|---|---|
| With tris(fluorosulphonyl)methane at 138℃; for 3h; | 100% |
| Hf[N(SO2C8F17)2]4 In various solvent(s) at 120℃; for 3h; | 98% |
| With C4F9SO3H at 138℃; for 5h; | 91% |
-
-
113860-02-9
[Cp*Ru(CH3CN)3]OTf

-
-
106-42-3
para-xylene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: CH3CN; under N2; addn. of Ru-complex to p-xylene and THF (benzene-free), mixt. stirred (30°C); addn. of hexane, solid filtered, washed twice (hexane), dried (vac.), elem. anal.; | 100% |
-
-
106-42-3
para-xylene

-
-
99-61-6
3-nitro-benzaldehyde

-
-
762-72-1
allyl-trimethyl-silane

-
-
1157865-90-1
tetrahydro-4-(2,5-dimethylphenyl)-2,6-bis(3-nitrophenyl)-2H-pyran

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate at 0 - 20℃; for 2h; Sakurai-Hosomi-Prins-Friedel-Crafts reaction; stereoselective reaction; | 100% |


| Conditions | Yield |
|---|---|
| at 40℃; for 480h; Gas phase; | 100% |

| Conditions | Yield |
|---|---|
| With (diethylamino)difluorosulfonium tetrafluoroborate In dichloromethane at 20℃; for 4h; Inert atmosphere; | 100% |
-
-
106-42-3
para-xylene

| Conditions | Yield |
|---|---|
| With methanesulfonic acid at 100℃; for 1h; | 100% |
-
-
106-42-3
para-xylene

-
-
66192-24-3
2-bromo-5-chlorobenzyl bromide

| Conditions | Yield |
|---|---|
| With indium(III) bromide In dichloromethane for 6h; Schlenk technique; Inert atmosphere; Molecular sieve; | 100% |

| Conditions | Yield |
|---|---|
| With ammonium acetate; water; hydrogen bromide; oxygen; manganese(II) acetate; cobalt(II) diacetate tetrahydrate; 1-n-butyl-3-methylimidazolim bromide; acetic acid at 200℃; under 30753.1 Torr; for 10h; Time; Temperature; Reagent/catalyst; Concentration; Inert atmosphere; | 99.9% |
| With oxovanadium(IV) sulfate; hydrogen bromide; oxygen; acetic acid In water at 100℃; under 750.075 Torr; for 20h; | 98% |
| With oxygen; acetic acid; hydrogen bromide; cobalt(II) acetate; manganese(II) acetate In water at 200℃; under 22502.3 Torr; for 0.05h; Product distribution / selectivity; Inert atmosphere; | 98.3% |

| Conditions | Yield |
|---|---|
| With chlorine; 3-butyl-1,2-dimethylimidazolium chloride In perfluoroheptane at 84℃; for 3h; Temperature; Solvent; | 99.11% |
| With chlorine; 3-butyl-1,2-dimethylimidazolium chloride at 120℃; for 3h; Reagent/catalyst; Temperature; Ionic liquid; Irradiation; | 99.1% |
| durch Chlorieren; |
-
-
106-42-3
para-xylene

| Conditions | Yield |
|---|---|
| Stage #1: para-xylene With 1-dodecyl-3-methylimidazol-1-ium chloride at 110℃; Irradiation; Stage #2: With chlorine at 90 - 120℃; Reagent/catalyst; Temperature; Reflux; | 99.11% |
-
-
91-01-0
1,1-Diphenylmethanol

-
-
106-42-3
para-xylene

-
-
7249-83-4
((2,5-dimethylphenyl)methylene)dibenzene

| Conditions | Yield |
|---|---|
| With silica gel supported sodium hydrogen sulfate at 80℃; for 0.5h; Friedel-Crafts type alkylation; | 99% |
| With H5CoW12O40 supported on rice husk ash extracted nano silica at 60℃; for 0.666667h; Reagent/catalyst; Green chemistry; | 98% |
| With Fe3O4/FeO at 60℃; for 0.333333h; Green chemistry; | 92% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; water at 20℃; for 10h; Inert atmosphere; | 99% |
| With N-hydroxy-tetrahydrophthalimide; oxygen; nitric acid at 50℃; under 750.075 - 1500.15 Torr; for 25h; Autoclave; Green chemistry; | 91.16% |
| With MoO(O2)(8-quinolinolate)2; dihydrogen peroxide In acetonitrile for 6h; Oxidation; Heating; | 88% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In chloroform at 0 - 20℃; Inert atmosphere; | 99% |
| With aluminum (III) chloride In carbon disulfide at 0 - 20℃; Friedel Crafts acylation; Inert atmosphere; | 95% |
| Friedel-Crafts alkylation; | 90% |

| Conditions | Yield |
|---|---|
| sulfated zirconia at 100℃; for 2h; Product distribution; Further Variations:; Catalysts; Friedel-Crafts acylation; | 99% |
| With C4F9SO3H at 138℃; for 5h; | 93% |

| Conditions | Yield |
|---|---|
| With indium(III) chloride; 4 A molecular sieve In dichloromethane at 20℃; for 16h; Friedel-Crafts alkylation; | 99% |
| With potassium cyanide; aluminum oxide at 50℃; for 5h; | 83 % Chromat. |
1,4-Dimethylbenzene Consensus Reports
1,4-Dimethylbenzene Standards and Recommendations
ACGIH TLV: TWA 100 ppm; STEL 150 ppm; BEI: methyl hippuric acids in urine at end of shift 1.5 g/g creatinine; Not Classifiable as a Human Carcinogen
NIOSH REL: (Xylene) TWA 100 ppm; CL 200 ppm/10M
1,4-Dimethylbenzene Specification
The 1,4-Dimethylbenzene, also called p-Xylene, is an aromatic hydrocarbon. It is an isomer of xylene. Other isomers include o-xylene and m-xylene. It occurs in two forms, either as colorless liquid with a characteristic aromatic odor at normal temperature, or as colorless crystalline solid below 13 °C. 1,4-Dimethylbenzene which exists in the atomatic tobacco and smoke is non-corrosive to metal. When heating to its decomposition, it decomposes to methane, hydrogen, toluene, couplet toluene and 2,6 - dimethyl anthracene.
Preparation: There is a considerable amount of 1,4-Dimethylbenzene in oil xylene and coal tar xylene. As the difference of boiling point between m-xylene and 1,4-Dimethylbenzene is only 0.75 °C, it can not be separated by distillation method. The current methods in domestic and foreign research are low-temperature crystallization separation, adsorption separation and complexing separation. The yield of low-temperature crystallization separation is 60-70% which is low. But the adsorption separation has relatively high yield and purity.
There is another method which has high purity of 99.9%. In transalkylation reactor, raw material toluene can produce xylene and benzene by alkyl transfer reaction. Part of m-xylene can be isomerized to 1,4-Dimethylbenzene by isomerization reactor. Then after some treatment, people can get the product with by-product m-xylene.
Uses: It is not only used as raw material in the production of polyester fibers and resins, paints, dyes and pesticides, but also used as chromatography standard materials and solvents. On a large scale, 1,4-Dimethylbenzene is used for the manufacture of terephthalic acid for polyester. In addition, it also can be used in organic synthesis. For example: it can react with benzoyl chloride to get 2,5-dimethyl-benzophenone. This reaction needs reagents CS2 and AlCl3.
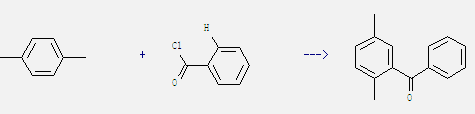
Safty: It has danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. If you want to contact this product, you must wear suitable protective clothing and gloves. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) So 1,4-Dimethylbenzene should be sealed in the container and stored in the cool and dry place which must be away from oxidant.
Other physical properties: (1)ACD/LogP: 3.25; (2)ACD/LogD (pH 5.5): 3.247; (3)ACD/LogD (pH 7.4): 3.247; (4)ACD/BCF (pH 5.5): 173.006; (5)ACD/BCF (pH 7.4): 173.006; (6)ACD/KOC (pH 5.5): 1391.912; (7)ACD/KOC (pH 7.4): 1391.912; (8)Index of Refraction: 1.5; (9)Molar Refractivity: 35.903 cm3; (10)Molar Volume: 121.984 cm3; (11)Surface Tension: 28.791 dyne/cm; (12)Density: 0.87 g/cm3; (13)Flash Point: 27.222 °C; (14)Enthalpy of Vaporization: 35.67 kJ/mol; (15)Boiling Point: 139.61 °C at 760 mmHg; (16)Vapour Pressure: 7.943 mmHg at 25°C.
Data structure conversion:
(1)Canonical SMILES: CC1=CC=C(C=C1)C
(2)InChI: InChI=1S/C8H10/c1-7-3-5-8(2)6-4-7/h3-6H,1-2H3
Toxicity:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LC | inhalation | > 8350ppm/4H (8350ppm) | BEHAVIORAL: TREMOR BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) BEHAVIORAL: ATAXIA | National Technical Information Service. Vol. OTS0556752, |
| mammal (species unspecified) | LDLo | intraperitoneal | 2gm/kg (2000mg/kg) | Journal of Pathology and Bacteriology. Vol. 46, Pg. 95, 1938. | |
| mammal (species unspecified) | LDLo | subcutaneous | 5gm/kg (5000mg/kg) | Journal of Pathology and Bacteriology. Vol. 46, Pg. 95, 1938. | |
| mouse | LCLo | inhalation | 15gm/m3 (15000mg/m3) | BEHAVIORAL: GENERAL ANESTHETIC LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION CARDIAC: OTHER CHANGES | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 143, Pg. 223, 1929. |
| mouse | LD50 | intraperitoneal | 2450uL/kg (2.45mL/kg) | Archives of Toxicology. Vol. 58, Pg. 106, 1985. | |
| rat | LC50 | inhalation | 4550ppm/4H (4550ppm) | LUNGS, THORAX, OR RESPIRATION: CHRONIC PULMONARY EDEMA LIVER: OTHER CHANGES BLOOD: CHANGES IN CELL COUNT (UNSPECIFIED) | "Biological Reactive Intermediates, Formation, Toxicity and Inactivation, Proceedings of the International Conference, Turku, Finland, 1975," Jollow, D.J., et al., eds., New York, Plenum Pub. Corp., 1977Vol. -, Pg. 302, 1977. |
| rat | LD50 | intraperitoneal | 3810mg/kg (3810mg/kg) | LUNGS, THORAX, OR RESPIRATION: CHRONIC PULMONARY EDEMA BLOOD: CHANGES IN CELL COUNT (UNSPECIFIED) LIVER: OTHER CHANGES | "Biological Reactive Intermediates, Formation, Toxicity and Inactivation, Proceedings of the International Conference, Turku, Finland, 1975," Jollow, D.J., et al., eds., New York, Plenum Pub. Corp., 1977Vol. -, Pg. 302, 1977. |
| rat | LD50 | oral | 5gm/kg (5000mg/kg) | Gekkan Yakuji. Pharmaceuticals Monthly. Vol. 22, Pg. 883, 1980. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 106424-71-9
- 106426-63-5
- 106428-05-1
- 106429-29-2
- 106-43-4
- 106436-39-9
- 106440-17-9
- 106-44-5
- 1064461-09-1
- 106447-41-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View