-
Name
2-Bromopyridine
- EINECS 203-641-6
- CAS No. 109-04-6
- Article Data65
- CAS DataBase
- Density 1.598 g/cm3
- Solubility Slightly miscible with water.
- Melting Point 193 °C
- Formula C5H4BrN
- Boiling Point 189.5 °C at 760 mmHg
- Molecular Weight 157.997
- Flash Point 54.4 °C
- Transport Information UN 2929 6.1/PG 2
- Appearance clear slight brown liquid
- Safety 16-36/37-45-38-36/37/39-28A-26
- Risk Codes 10-24/25-36/37/38-23/24/25-20/21/22
-
Molecular Structure
-
Hazard Symbols
 T,
T, Xn,
Xn, F
F
- Synonyms beta-Bromopyridine;o-Bromopyridine;PYRIDINE,2-BROMO;.beta.-Bromopyridine;Pyridine, 2-bromo-;2-Pyridyl bromide;2-Bromopuridine;2 - Bromo Pyridine;2-Bromo Pyridine;2- Bromopyridine;
- PSA 12.89000
- LogP 1.84410
Synthetic route

| Conditions | Yield |
|---|---|
| With n-butyllithium; butyl magnesium bromide In toluene at -10℃; | 100% |
| With n-butyllithium; isopropyl alcohol In hexane; dichloromethane 1.) -78 deg C, 1 h, 2.) -78 deg C, 30, min; warm to room temperature; | 90% |
| With n-butyllithium; formaldehyd In tetrahydrofuran at -70 - -40℃; | 90% |
-
-
80866-91-7
2-bromopyridine 1-oxide hydrochloride

-
-
109-04-6
2-bromo-pyridine

| Conditions | Yield |
|---|---|
| With titanium In tetrahydrofuran for 0.25h; Ambient temperature; | 97% |
-
-
14305-17-0
2-bromopyridine-N-oxide

-
-
109-04-6
2-bromo-pyridine

| Conditions | Yield |
|---|---|
| With Dimethylphenylsilane In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 96% |
| With triphenylphosphine; [MoO2Cl2(dmf)2] In tetrahydrofuran for 5h; Heating; | 87% |
| With hydrogen; triethyl phosphite In isopropyl alcohol at 100℃; under 4500.45 Torr; for 12h; Glovebox; chemoselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| With 2-methylphenyl bromide; copper(I) bromide In cyclohexane at 5 - 45℃; for 3.41667h; Concentration; Temperature; | 91% |
| 78% | |
| With hydrogen bromide; bromine; sodium nitrite In water at -2℃; for 0.333333h; | 65% |

| Conditions | Yield |
|---|---|
| With bromine In cyclohexane at 0 - 5℃; for 1h; | A 86% B 10% |
| With bromine In cyclohexane at 75℃; for 1h; | A 32% B 66% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; phosphorus pentoxide In 1,2-dichloro-benzene at 180℃; for 10h; | 75% |
| With N-Bromosuccinimide; triphenylphosphine In 1,4-dioxane for 4h; Bromination; substitution; Heating; | 54% |
| With carbon tetrabromide; triphenylphosphine In toluene for 8h; Reflux; | 100 %Chromat. |

| Conditions | Yield |
|---|---|
| With n-butyllithium; 2-(N,N-dimethylamino)ethanol; carbon tetrabromide 1.) hexane, -78 deg C, 1 h, 2.) THF, -78 deg C, 1 h; | 72% |
-
-
624-28-2
2,5-dibromopyridine

-
-
96-22-0
pentan-3-one

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
874915-22-7
2-(3-hydroxy-pentane-3-yl)-5-bromo-pyridine

-
C
-
874915-24-9
2-bromo-5-(3-hydroxy-pentane-3-yl)pyridine

-
D
-
19780-41-7
3-ethylheptan-3-ol

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at 5℃; for 0.0833333h; | A 8.7% B 11% C 68.8% D 3.9% |

-
-
624-28-2
2,5-dibromopyridine

-
-
96-22-0
pentan-3-one

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
874915-22-7
2-(3-hydroxy-pentane-3-yl)-5-bromo-pyridine

-
C
-
874915-24-9
2-bromo-5-(3-hydroxy-pentane-3-yl)pyridine

-
D
-
874915-27-2
2,5-di(3-hydroxy-pentane-3-yl)pyridine

-
E
-
19780-41-7
3-ethylheptan-3-ol

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at 5℃; for 0.1h; | A 2.8% B 1.9% C 60.7% D 2.4% E 4.8% |

-
-
110-86-1
pyridine

-
-
78948-09-1
acetyl hypofluorite

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
3847-19-6
2-acetoxypyridine

| Conditions | Yield |
|---|---|
| With 1,2-dibromomethane Ambient temperature; | A 60% B 20% |


| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; triphenylphosphine In 1,4-dioxane for 4h; Heating; | 54% |

| Conditions | Yield |
|---|---|
| With trimethylsilyl bromide In various solvent(s) for 100h; Heating; | 49% |

| Conditions | Yield |
|---|---|
| In toluene at 20℃; for 3h; Inert atmosphere; Schlenk technique; | A n/a B 43% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; n-butyllithium; 2-(N,N-dimethylamino)ethanol 1.) hexane, -78 deg C, 1 h, 2.) THF, -78 deg C, 1 h; | A 45 % Chromat. B 25% |


| Conditions | Yield |
|---|---|
| at 0 - 20℃; for 2.5h; Inert atmosphere; Glovebox; | A n/a B 22% |


| Conditions | Yield |
|---|---|
| With bromine at 500℃; Leiten ueber Bimsstein; | |
| With bromine at 500℃; Leiten ueber Glas; |

| Conditions | Yield |
|---|---|
| With methanol; n-butyllithium 1.) THF, -89 deg C, 4.5 h, 2.) THF, from -90 deg C to RT, 6 h; Multistep reaction; |
-
-
52200-48-3
3-bromo-2-chloropyridine

-
-
109-72-8, 29786-93-4
n-butyllithium

-
A
-
109-09-1
2-chloropyridine

-
B
-
109-04-6
2-bromo-pyridine

-
C
-
100921-62-8
bromo-4 chloro-2 deuterio-3 pyridine

-
D
-
73583-36-5
chloro-2 butyl-3 pyridine

-
E
-
100921-61-7
chloro-2 deuterio-3 pyridine

-
F
-
100921-63-9
chloro-2 butyl-4 pyridine

| Conditions | Yield |
|---|---|
| With ethyl [2]alcohol Product distribution; Mechanism; 1) THF, -40 deg C, 10 min, 2) THF, -40 deg C, 15 min; a new "homotransmetalation" reaction, further reagents and conditions; |

-
-
52200-48-3
3-bromo-2-chloropyridine

-
-
109-72-8, 29786-93-4
n-butyllithium

-
A
-
109-09-1
2-chloropyridine

-
B
-
109-04-6
2-bromo-pyridine

-
C
-
100921-62-8
bromo-4 chloro-2 deuterio-3 pyridine

-
D
-
73589-41-0
chloro-2 bromo-4 butyl-3 pyridine

| Conditions | Yield |
|---|---|
| With ethyl [2]alcohol 1) THF, -40 deg C, 15 min, 2) THF, -40 deg C, 15 min; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
52200-48-3
3-bromo-2-chloropyridine

-
-
109-72-8, 29786-93-4
n-butyllithium

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
100921-62-8
bromo-4 chloro-2 deuterio-3 pyridine

-
C
-
73583-36-5
chloro-2 butyl-3 pyridine

-
D
-
100921-63-9
chloro-2 butyl-4 pyridine

| Conditions | Yield |
|---|---|
| With ethyl [2]alcohol 1) THF, -40 deg C, 10 min, 2) THF, -40 deg C, 15 min; Yield given. Multistep reaction. Further byproducts given. Yields of byproduct given; |

-
-
52200-48-3
3-bromo-2-chloropyridine

-
A
-
109-09-1
2-chloropyridine

-
B
-
109-04-6
2-bromo-pyridine

-
C
-
100921-62-8
bromo-4 chloro-2 deuterio-3 pyridine

-
D
-
73589-41-0
chloro-2 bromo-4 butyl-3 pyridine

| Conditions | Yield |
|---|---|
| With n-butyllithium; ethyl [2]alcohol 1) THF, -40 deg C, 15 min, 2) THF, -40 deg C, 15 min; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
52200-48-3
3-bromo-2-chloropyridine

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
100921-62-8
bromo-4 chloro-2 deuterio-3 pyridine

-
C
-
73583-36-5
chloro-2 butyl-3 pyridine

-
D
-
100921-63-9
chloro-2 butyl-4 pyridine

| Conditions | Yield |
|---|---|
| With n-butyllithium; ethyl [2]alcohol 1) THF, -40 deg C, 10 min, 2) THF, -40 deg C, 15 min; Yield given. Multistep reaction. Further byproducts given. Yields of byproduct given; |

-
-
109-65-9
1-bromo-butane

-
-
13534-89-9
2,3-dibromopyridine

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
100921-66-2
bromo-2 butyl-3 pyridine

| Conditions | Yield |
|---|---|
| 1) THF, -60 deg C, 15 min, 2) THF, -60 deg C, 1 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
109-65-9
1-bromo-butane

-
-
13534-89-9
2,3-dibromopyridine

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
100921-68-4
dibromo-2,3 butyl-4 pyridine

-
C
-
100921-67-3
dibromo-2,4 butyl-3 pyridine

| Conditions | Yield |
|---|---|
| 1) THF, -60 to -40 deg C, 10 min, 2) THF, -60 deg C, 1 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
13534-89-9
2,3-dibromopyridine

-
-
109-72-8, 29786-93-4
n-butyllithium

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
54267-61-7
bromo-2 deuterio-3 pyridine

-
C
-
100933-54-8
bromo-2 butyl-4 pyridine

-
D
-
100921-65-1
dibromo-2,4 deuterio-3 pyridine

| Conditions | Yield |
|---|---|
| With ethyl [2]alcohol Product distribution; Mechanism; 1) THF, -40 deg C, 10 min, 2) THF, -40 deg C, 15 min; a new "homotransmetalation" reaction, further reagents and conditions; | |
| With ethyl [2]alcohol 1) THF, -40 deg C, 10 min, 2) THF, -40 deg C, 15 min; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
13534-89-9
2,3-dibromopyridine

-
-
96-22-0
pentan-3-one

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
73583-40-1
bromo-2 (ethyl-1 hydroxy-1 propyl)-3 pyridine

| Conditions | Yield |
|---|---|
| With n-butyllithium 1) THF, -60 deg C, 15 min, 2) THF, -60 deg C, 15 min; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
13534-89-9
2,3-dibromopyridine

-
-
96-22-0
pentan-3-one

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
100921-64-0
dibromo-2,3 (ethyl-1 hydroxy-1 propyl)-4 pyridine

| Conditions | Yield |
|---|---|
| With n-butyllithium 1) THF, -60 to -40 deg C, 20 min, 2) THF, -60 deg C, 30 min; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With n-butyllithium; pentan-3-one 1) THF, -40 deg C, 15 min, 2) THF, -60 deg C, 15 min; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
13534-89-9
2,3-dibromopyridine

-
A
-
109-04-6
2-bromo-pyridine

-
B
-
54267-61-7
bromo-2 deuterio-3 pyridine

-
C
-
100921-65-1
dibromo-2,4 deuterio-3 pyridine

| Conditions | Yield |
|---|---|
| With n-butyllithium; ethyl [2]alcohol 1) THF, -40 deg C, 15 min, 2) THF, -40 deg C, 15 min; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With copper; caesium carbonate In N,N-dimethyl-formamide at 100℃; for 0.166667h; Ullmann Condensation; Microwave irradiation; Inert atmosphere; | 100% |
| With 1,1,1,5,5,5-hexafluoroacetylacetone; copper(II) ferrite; caesium carbonate In 1-methyl-pyrrolidin-2-one at 135℃; for 24h; Ullmann Condensation; Inert atmosphere; Schlenk technique; | 99% |
| With 2-acetonylpyridine; caesium carbonate; copper(I) bromide In dimethyl sulfoxide at 90℃; for 15h; Inert atmosphere; chemoselective reaction; | 98% |

| Conditions | Yield |
|---|---|
| With [Cu2(2,7-bis(pyridin-2-yl)-l,8-naphthyridine)(OH)(CF3COO)3]; tetrabutylammomium bromide; ammonia; caesium carbonate In water at 110 - 120℃; for 16h; Sealed tube; | 100% |
| With ammonia; triethylamine In water at 20℃; for 4.25h; | 97% |
| With ammonium hydroxide at 20℃; for 7h; Catalytic behavior; | 96% |

| Conditions | Yield |
|---|---|
| With hydrazine at 100℃; for 2h; Substitution; | 100% |
| With hydrazine hydrate In ethanol; water | 58% |
| With hydrazine hydrate for 4h; Heating; | 54% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate In water; N,N-dimethyl-formamide at 210℃; for 24h; Catalytic behavior; Solvent; Reagent/catalyst; | 100% |
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; cesium fluoride In dimethyl sulfoxide at 120℃; for 8h; | 96% |
| With bis(triphenylphosphine)nickel(II) chloride; sodium hydride; triphenylphosphine; zinc In toluene at 70 - 90℃; Ullmann-type coupling; | 93% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine In toluene for 24h; Inert atmosphere; Reflux; | 100% |
| With caesium carbonate; copper(I) bromide In dimethyl sulfoxide at 60℃; for 22h; | 95% |
| With dimethylenecyclourethane; copper(l) iodide; sodium methylate In dimethyl sulfoxide at 120℃; for 24h; | 89% |
| With sodium hydride In N,N-dimethyl-formamide at 130℃; for 0.5h; microwave irradiation; | 65% |
| With potassium acetate; CuO/SiO2 In xylene for 18h; Heating; | 49% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide | 100% |
-
-
109-04-6
2-bromo-pyridine

-
-
16419-60-6
2-Methylphenylboronic acid

-
-
10273-89-9
2-(2-methylphenyl)pyridine

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 18h; Inert atmosphere; Reflux; | 100% |
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In water; N,N-dimethyl-formamide at 120℃; for 12h; Suzuki Coupling; Inert atmosphere; | 96% |
| With potassium phosphate tribasic heptahydrate; palladium diacetate In ethylene glycol at 80℃; for 1h; Suzuki coupling; | 94% |
-
-
109-04-6
2-bromo-pyridine

-
-
16750-63-3
2-methoxyphenylmagnesium bromide

-
-
5957-89-1
2-(2-methoxy-phenyl)-pyridine

| Conditions | Yield |
|---|---|
| With 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) In tetrahydrofuran at 0 - 20℃; | 100% |
| With tetrakis(triphenylphosphine) palladium(0) In toluene at 50 - 100℃; for 1h; | 89% |
| (1,2-bis(diphenylphosphanyl)ethane)dichloridopalladium(II) In tetrahydrofuran for 12h; Ambient temperature; | 87% |
| With bis(acetylacetonate)nickel(II); 1,2-bis-(diphenylphosphino)ethane In tetrahydrofuran at 20℃; for 18h; Corriu-Kumada-Tamao coupling; | 80% |
| With nickel catalyst Inert atmosphere; |
-
-
109-04-6
2-bromo-pyridine

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
5683-33-0
2-(Dimethylamino)pyridine

| Conditions | Yield |
|---|---|
| With potassium hydroxide | 100% |
| at 130℃; for 12h; | 92% |
| With potassium hydroxide In neat (no solvent) at 100℃; for 24h; Sealed tube; | 41% |

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine In toluene at 60℃; for 16h; Sonogashira coupling; | 100% |
| With potassium carbonate; triphenylphosphine; silver(I) iodide In N,N-dimethyl-formamide at 100℃; for 8h; Sonogashira coupling reaction; | 99% |
| With tetrabutylammomium bromide; potassium acetate; cyclopalladated ferrocenylimine In N,N-dimethyl acetamide at 80℃; for 20h; Sonogashira coupling; | 99% |
-
-
109-04-6
2-bromo-pyridine

-
-
1066-54-2
trimethylsilylacetylene

-
-
86521-05-3
2-(trimethylsilylethynyl)pyridine

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 70℃; for 3h; | 100% |
| With copper(l) iodide; tetrakis(triphenylphosphine) palladium(0); triethylamine at 20℃; | 97% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 50℃; Sonogashira Cross-Coupling; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With [Pd(N-(naphthyl)-salicylaldimine(-2H))(triphenylphosphine)]; sodium hydroxide at 120℃; for 24h; Reagent/catalyst; Suzuki Coupling; | 100% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 18h; Inert atmosphere; Reflux; | 100% |
| With potassium phosphate; (PdCl2)(2-pyridyl)-6-isopropyl cis piperidine complex In water; N,N-dimethyl-formamide at 110℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; triphenylphosphine; bis-triphenylphosphine-palladium(II) chloride In triethylamine at 25℃; for 16h; Sonogashira coupling; | 100% |
| Stage #1: 2-bromo-pyridine With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 25℃; for 0.5h; Inert atmosphere; Stage #2: 1-Pentyne at 25℃; for 16h; Inert atmosphere; | 94% |
| Stage #1: 2-bromo-pyridine With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine at 25℃; for 0.5h; Inert atmosphere; Stage #2: 1-Pentyne at 25℃; for 16h; Inert atmosphere; | 94% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide In diethylamine for 20h; Ambient temperature; | 84% |
| Stage #1: 2-bromo-pyridine With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine; triphenylphosphine for 0.5h; Sonogashira Cross-Coupling; Inert atmosphere; Stage #2: 1-Pentyne for 18h; Sonogashira Cross-Coupling; Inert atmosphere; Darkness; | 78% |
-
-
109-04-6
2-bromo-pyridine

-
-
24856-58-4
1,1-dimethoxy-1-(4-bromophenyl)methane

-
-
127406-56-8
2-(4-formylphenyl)pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 1,1-dimethoxy-1-(4-bromophenyl)methane With iodine; magnesium In tetrahydrofuran at 30 - 35℃; for 2h; Stage #2: 2-bromo-pyridine; tetrakis(triphenylphosphine) palladium(0); zinc(II) chloride In tetrahydrofuran; toluene at 50℃; for 2.5h; Stage #3: With hydrogenchloride; ammonia; water Conversion of starting material; more than 3 stages; | 100% |
| Stage #1: 1,1-dimethoxy-1-(4-bromophenyl)methane With iodine; magnesium In tetrahydrofuran at 30 - 35℃; for 2.5h; Stage #2: 2-bromo-pyridine; tetrakis(triphenylphosphine) palladium(0); zinc(II) chloride In tetrahydrofuran at 70℃; for 3.5h; Stage #3: With hydrogenchloride; ammonia; water Conversion of starting material; more than 3 stages; | 99% |
| Stage #1: 1,1-dimethoxy-1-(4-bromophenyl)methane With iodine; magnesium In tetrahydrofuran at 30 - 35℃; for 2.5h; Stage #2: 2-bromo-pyridine; tetrakis(triphenylphosphine) palladium(0); zinc(II) chloride In tetrahydrofuran at 50℃; for 3.5h; Stage #3: With hydrogenchloride; ammonia; water Conversion of starting material; more than 3 stages; | 96.4% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; CVT-2537 In dimethyl sulfoxide at 90℃; for 24h; Inert atmosphere; | 100% |
| With 1-methyl-3-(2-pyridinyl)-3,4,5,6-tetrahydropyrimidin-3-ium hexafluorophosphate; potassium tert-butylate; palladium diacetate In 1,2-dimethoxyethane at 100℃; for 0.666667h; Buchwald-Hartwig Coupling; Microwave irradiation; | 97% |
| at 120℃; for 0.333333h; microwave irradiation; | 92% |
-
-
109-04-6
2-bromo-pyridine

-
-
1993-03-9
o-fluorophenylboronic acid

-
-
89346-48-5
2-(2-fluorophenyl)pyridine

| Conditions | Yield |
|---|---|
| With trans-bis(triphenylphosphine)palladium dichloride; potassium carbonate In 1,2-dimethoxyethane; water at 100℃; | 100% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 12h; Suzuki-Miyaura Coupling; Inert atmosphere; Reflux; | 92% |
| With bis-triphenylphosphine-palladium(II) chloride; potassium carbonate In 1,2-dimethoxyethane; water at 80℃; for 12h; Suzuki-Miyaura Coupling; | 90% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In toluene at 120℃; for 24h; Ullmann Condensation; Sealed tube; | 100% |
| With copper(l) iodide; caesium carbonate In 4-methyl-2-pentanone at 120℃; for 24h; Ullmann type condensation; Inert atmosphere; | 98% |
| With potassium phosphate; copper(l) iodide; (1S,2S,N1E,N2E)-N,N'-bis(pyridin-2-ylmethylene)cyclohexane-1,2-diamine In acetonitrile at 80℃; for 24h; Ullmann reaction; | 97% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; tetrakis(triphenylphosphine) palladium(0); N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran at 55℃; for 10h; | 100% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium(0) chloroform complex; 1,1'-bis-(diphenylphosphino)ferrocene In 1,2-dimethoxyethane; n-heptane at 85℃; for 0.5h; | 100% |
-
-
109-04-6
2-bromo-pyridine

-
-
771565-23-2
N-[1-(4-methoxyphenyl)prop-2-yn-1-yl]-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With copper(l) iodide; triethylamine; bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran Heating; | 100% |
-
-
109-04-6
2-bromo-pyridine

-
-
848775-01-9
[(3-phenyl-7,8,9,10-tetrahydro-7,10-ethano[1,2,4]triazolo[3,4-a]phthalazin-6-yl)methyl]amine

| Conditions | Yield |
|---|---|
| at 160℃; for 6h; | 100% |
-
-
109-04-6
2-bromo-pyridine

-
-
149104-90-5
4-acetylphenylboronic acid

-
-
173681-56-6
1-(4-(pyridin-2-yl)phenyl)ethanone

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 18h; Inert atmosphere; Reflux; | 100% |
| With [(t-Bu)3PH]BF4; palladium diacetate; sodium hydroxide at 20℃; for 0.25h; Suzuki Coupling; Inert atmosphere; Glovebox; | 90% |
| With potassium phosphate In ethanol; water at 100℃; for 8h; Suzuki-Miyaura Coupling; | 82% |
-
-
109-04-6
2-bromo-pyridine

-
-
227178-09-8
1-(3-(pyridin-2-yl)phenyl)ethan-1-one

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate; triphenylphosphine In 1,2-dimethoxyethane; water for 15h; Reflux; Inert atmosphere; Schlenk technique; | 100% |
| With sodium carbonate; triphenylphosphine; palladium diacetate In propan-1-ol Suzuki-Miyaura cross-coupling; Heating; | |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 12h; Inert atmosphere; Reflux; | |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene at 95℃; for 18h; | |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 12h; Inert atmosphere; Schlenk technique; Reflux; |
-
-
109-04-6
2-bromo-pyridine

-
-
144025-03-6
2,4-difluorophenylboronic acid

-
-
391604-55-0
2-(2,4-difluorophenyl)pyridine

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In tetrahydrofuran at 70℃; for 24h; Suzuki coupling; | 100% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In tetrahydrofuran; water at 70℃; Inert atmosphere; | 98% |
| With sodium carbonate; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran; water at 70℃; for 24h; | 97% |
-
-
109-04-6
2-bromo-pyridine

-
-
87199-17-5
4-formylphenylboronic acid,

-
-
127406-56-8
2-(4-formylphenyl)pyridine

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In 1,4-dioxane; water for 8h; Suzuki coupling; Reflux; | 100% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 18h; Inert atmosphere; Reflux; | 100% |
| Stage #1: 2-bromo-pyridine; 4-formylphenylboronic acid, With potassium carbonate In 1,4-dioxane; water for 0.5h; Inert atmosphere; Stage #2: With tetrakis(triphenylphosphine) palladium(0) In 1,4-dioxane; water Inert atmosphere; Reflux; | 100% |
-
-
109-04-6
2-bromo-pyridine

-
-
192887-50-6
(4-methoxyphenyl)manganese(II) chloride

-
-
5957-90-4
4-(2-pyridinyl)anisole

| Conditions | Yield |
|---|---|
| Stage #1: (4-methoxyphenyl)manganese(II) chloride With 1,3-bis[2,6-bis(1-methylethyl)phenyl]-1,3-dihydro-2H-imidazol-2-ylidene monohydrochloride; bis(acetylacetonate)nickel(II) In tetrahydrofuran at 0℃; for 0.5h; Stage #2: 2-bromo-pyridine In tetrahydrofuran at 0 - 25℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 18h; Inert atmosphere; Reflux; | 100% |
| With bis-triphenylphosphine-palladium(II) chloride; sodium carbonate In water; isopropyl alcohol at 80℃; for 0.666667h; Suzuki Coupling; Microwave irradiation; | 91% |
| With bis(triphenylphosphine)palladium(II) dichloride; potassium carbonate In 1,2-dimethoxyethane; water at 80℃; for 14h; | 86% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 12h; Inert atmosphere; Reflux; | 100% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; water; toluene for 18h; Inert atmosphere; Reflux; | 100% |
| With potassium phosphate tribasic heptahydrate; palladium diacetate In isopropyl alcohol at 80℃; for 0.2h; Suzuki coupling; | 99% |

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; copper(l) iodide In toluene at 160℃; for 16h; Product distribution / selectivity; | 100% |
| With 1-methyl-1H-imidazole; copper(l) iodide at 140℃; for 16h; Product distribution / selectivity; | 100% |
| With 1,3-bis-(diphenylphosphino)propane; sodium carbonate; palladium diacetate In 1-methyl-pyrrolidin-2-one at 130℃; for 16h; Product distribution / selectivity; | 30% |
2-Bromopyridine Chemical Properties
Molecular structure of 2-Bromopyridine (CAS NO.109-04-6) is:
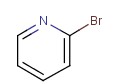
Product Name: 2-Bromopyridine
CAS Registry Number: 109-04-6
IUPAC Name: 2-bromopyridine
Molecular Weight: 157.99596 [g/mol]
Molecular Formula: C5H4BrN
XLogP3: 1.4
H-Bond Donor: 0
H-Bond Acceptor: 1
EINECS: 203-641-6
Melting Point:193 °C
Surface Tension: 42.1 dyne/cm
Density: 1.598 g/cm3
Flash Point: 54.4 °C
Enthalpy of Vaporization: 40.83 kJ/mol
Boiling Point: 189.5 °C at 760 mmHg
Vapour Pressure: 0.784 mmHg at 25°C
Refractive index: n20/D 1.572(lit.)
Storage temp.: Refrigerator
Stability: Stable. Flammable. Incompatible with strong oxidizing agents, strong acids, acid chlorides.
Product Categories: Pyridine;Pyridines, Pyrimidines, Purines and Pteredines;Halides;Pyridines;Pyridines derivates;Bromopyridines;Halopyridines;C5Heterocyclic Building Blocks;Halogenated Heterocycles;Heterocyclic Building Blocks
2-Bromopyridine Uses
2-Bromopyridine (CAS NO.109-04-6) is used as organic synthesis, pharmaceutical intermediates. In the pharmaceutical industry, it is used for the synthesis of heart drugs called dual-isopropyl amine topiramate and so on.
2-Bromopyridine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | intraperitoneal | 31300ug/kg (31.3mg/kg) | "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 4, Pg. 322, 1952. |
2-Bromopyridine Safety Profile
Hazard Codes:  T,
T,  Xn,
Xn,  F
F
Risk Statements: 10-24/25-36/37/38-23/24/25-20/21/22
R26/27/28:Very toxic by inhalation, in contact with skin and if swallowed.
R23/25:Toxic by inhalation and if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
Safety Statements: 16-36/37-45-38-36/37/39-28A-26
S16:Keep away from sources of ignition.
S36/37:Wear suitable protective clothing and gloves.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S38:In case of insufficient ventilation, wear suitable respiratory equipment.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S28:After contact with skin, wash immediately with plenty of soap-suds.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
RIDADR: UN 2929 6.1/PG 2
WGK Germany: 3
RTECS: US3850000
F: 8
Hazard Note: Toxic
HazardClass: 6.1
PackingGroup: II
HS Code: 29333999
2-Bromopyridine Specification
2-Bromopyridine , its cas register number is 109-04-6. It also can be called 2-Pyridyl bromide ; beta-Bromopyridine ; o-Bromopyridine ; Pyridine, 2-bromo- .It is a dark red liquid.
Related Products
- 2-Bromopyridine
- 2-Bromopyridine N-oxide hydrobromide
- 2-Bromopyridine N-oxide hydrochloride
- 2-Bromopyridine-3-boronic acid
- 2-Bromopyridine-3-boronic acid pinacol ester
- 2-Bromopyridine-3-carbaldehyde
- 2-Bromopyridine-4-boronic acid
- 2-Bromopyridine-4-boronic acid pinacol ester
- 2-Bromopyridine-4-methanamine
- 2-Bromopyridine-4-methanol
- 109049-12-9
- 109056-69-1
- 109056-72-6
- 109-05-7
- 1090587-89-5
- 1090-65-9
- 109-06-8
- 109069-75-2
- 109073-77-0
- 109074-67-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View