-
Name
2-Chlorobenzonitrile
- EINECS 212-836-5
- CAS No. 873-32-5
- Article Data257
- CAS DataBase
- Density 1.23 g/cm3
- Solubility
- Melting Point 43-46 °C(lit.)
- Formula C7H4ClN
- Boiling Point 232.8 °C at 760 mmHg
- Molecular Weight 137.568
- Flash Point 100.8 °C
- Transport Information UN 3439
- Appearance white to light yellow crystal powder
- Safety 23
- Risk Codes 21/22-36-21/22/23
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Benzonitrile, 2-chloro-;Benzonitrile, o-chloro-;o-Chlorbenzonitril [Czech];o-Cyanochlorobenzene;o-Chlorocyanobenzene;Nitril kyseliny o-chlorbenzoove [Czech];2-Chloro-Benzonitrile;2-chloro benzonitrile;o-chlorotolunitrile;
- PSA 23.79000
- LogP 2.21168
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; sodium persulfate; sodium iodide; iron(II) chloride In 1,2-dichloro-ethane at 20 - 50℃; for 16h; | 99% |
| With copper(II) oxide; hydroxylamine hydrochloride In neat (no solvent) for 0.0208333h; Mechanism; Reagent/catalyst; Microwave irradiation; Green chemistry; | 96% |
| With ammonium hydroxide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen In ethanol at 40℃; under 1500.15 - 3000.3 Torr; for 4h; | 96% |
-
-
17849-38-6
2-Chlorobenzyl alcohol

-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 2-Chlorobenzyl alcohol With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tert-butylhypochlorite In dichloromethane at 20℃; for 0.5h; Inert atmosphere; Stage #2: With ammonia; iodine In dichloromethane; water at 20℃; for 2h; Inert atmosphere; | 99% |
| With ammonium hydroxide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen In ethanol at 40℃; under 1500.15 - 3000.3 Torr; for 4h; Reagent/catalyst; | 97.1% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; ammonia; oxygen; copper(II) nitrate In water; dimethyl sulfoxide at 80℃; under 760.051 Torr; for 7h; | 95% |
-
-
15717-17-6
2-chlorothiobenzamide

-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| With polystyrene-bound diaryl telluroxide In dichloromethane for 1h; Ambient temperature; | 98% |
| With polystyrne-bound selenoxide In ethanol for 24h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With pyridine; Oxone; 4-acetylamino-2,2,6,6-tetramethyl-1-piperidinoxy; Pyridine hydrobromide In dichloromethane at 20℃; for 12h; Green chemistry; | 98% |
| With C68H64Cl2N6P2Ru2(4+)*2F6P(1-)*2Cl(1-); caesium carbonate In N,N-dimethyl-formamide at 100℃; for 24h; Inert atmosphere; Green chemistry; | 91.9% |
| With 2,2,6,6-tetramethyl-piperidine-N-oxyl; free radical; trichloroisocyanuric acid In dichloromethane at 10℃; for 2h; | 90% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(dodecylthio)benzonitrile With sulfuryl dichloride; water In chlorobenzene at 20 - 65℃; for 3h; Stage #2: With sodium hydroxide In water at 60 - 65℃; pH=9 - 10; Stage #3: With hydrogenchloride In water at 20 - 30℃; pH=3 - 4; Reagent/catalyst; | A 106 g B 97.2% C 143 g |


| Conditions | Yield |
|---|---|
| With N-trifluoroacetylimidazole In tetrahydrofuran for 3.5h; Heating; | 97% |
| With cerium(IV) oxide In o-xylene at 160℃; for 0.5h; Dean-Stark; Inert atmosphere; | 97% |
| With phosphoric acid diethyl ester 2-phenylbenzimidazol-1-yl ester; triethylamine In acetonitrile at 20℃; for 0.416667h; | 96% |

| Conditions | Yield |
|---|---|
| at 350℃; under 48754.9 Torr; for 0.416667h; Supercritical conditions; Flow reactor; | 97% |
| With molybdenum hexacarbonyl In tetrachloromethane at 150℃; for 6h; Autoclave; | 28% |

-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| With iron(III) sulfate; 4-nitro-phenol In cyclohexane at 20℃; for 0.116667h; | 97% |
| With iron(III) chloride; 2,6-di-tert-butyl-4-methyl-phenol In toluene at 20℃; for 0.116667h; Schlenk technique; | 86% |

| Conditions | Yield |
|---|---|
| With trimethylsilyl methanesulfonate; phosphorus pentoxide at 70 - 75℃; for 3h; further reagents; | 95% |
| With triethylamine; trifluoroacetyl chloride In dichloromethane | 93% |
| With C8H14N2O4S In dichloromethane for 1h; Reflux; | 93% |
-
-
17849-38-6
2-Chlorobenzyl alcohol

-
-
89-98-5
2-chloro-benzaldehyde

-
-
118-91-2
ortho-chlorobenzoic acid

-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen In ethanol at 40℃; under 1500.15 - 3000.3 Torr; for 4h; | 95% |


| Conditions | Yield |
|---|---|
| With ammonium hydroxide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; oxygen In ethanol at 40℃; under 1500.15 - 3000.3 Torr; for 4h; | 94.5% |
| With hydroxyammonium sulfate; zinc for 0.416667h; Microwave irradiation; | 90% |
-
-
1160509-17-0
2-cyanophenyl octyl sulfide

-
A
-
2634-33-5
1,2-benzisothiazolin-3-one

-
B
-
873-32-5
2-Chlorobenzonitrile

-
C
-
111-85-3
1-Chlorooctane

| Conditions | Yield |
|---|---|
| Stage #1: 2-cyanophenyl octyl sulfide With water; chlorine In chlorobenzene at 20 - 65℃; for 3h; Stage #2: With sodium hydroxide In water at 60 - 65℃; pH=9 - 10; Stage #3: With hydrogenchloride In water at 20 - 30℃; pH=3 - 4; | A 103 g B 94.5% C 100 g |


| Conditions | Yield |
|---|---|
| With potassium phenolate; palladium diacetate; potassium carbonate; dimethyl cis-but-2-ene-1,4-dioate; triphenylphosphine In N,N-dimethyl-formamide at 120℃; for 24h; Schlenk technique; Inert atmosphere; | 94% |

-
A
-
2634-33-5
1,2-benzisothiazolin-3-one

-
B
-
873-32-5
2-Chlorobenzonitrile

-
C
-
3386-33-2
1-chlorooctadecane

| Conditions | Yield |
|---|---|
| Stage #1: 2-(octadecylthio)benzonitrile With water; chlorine In chlorobenzene at 20 - 65℃; for 3h; Stage #2: With sodium hydroxide In water at 60 - 65℃; pH=9 - 10; Stage #3: With hydrogenchloride In water at 20 - 30℃; pH=3 - 4; | A 102 g B 93.5% C 210 g |


-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 0.5h; Elimination; Heating; | 93% |

| Conditions | Yield |
|---|---|
| With oxygen; copper(II) trifluoroacetate; urea In dimethyl sulfoxide at 110℃; for 26h; Green chemistry; | 93% |
| With iron(III) trifluoromethanesulfonate; sodium nitrite In dimethyl sulfoxide at 50℃; for 10h; Inert atmosphere; Sealed tube; | 46% |

| Conditions | Yield |
|---|---|
| With mesoporous silica SBA-15 supported Cu2O nanoparticles In N,N-dimethyl-formamide at 120℃; for 8h; Green chemistry; | 93% |
| With 3% Pd/CeO2; sodium acetate In N,N-dimethyl-formamide; isopropyl alcohol at 55℃; for 12h; Irradiation; | 26% |
-
-
62717-50-4
o-chlorostyrene oxide

-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| With ammonia; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In water; acetonitrile at 70℃; | 91% |
| With ammonia; iodine In water; acetonitrile at 70℃; | 83% |

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; palladium diacetate; toluene-4-sulfonic acid In 1,2-dichloro-ethane at 70℃; for 12h; Sealed tube; | 90% |
-
-
615-41-8
2-iodochlorobenzene

-
-
19555-13-6
2-(dimethylamino)malononitrile

-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| With copper(II) orthophosphate In N,N-dimethyl-formamide at 120℃; for 24h; Inert atmosphere; Sealed tube; | 90% |
-
-
3717-28-0
2-chloro benzaldehyde oxime

-
-
76-02-8
Trichloroacetyl chloride

-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 1h; Ambient temperature; | 88% |

| Conditions | Yield |
|---|---|
| With [Ru(η6-C6Me6)Cl2(tris(dimethylamino)phosphine)]; hydroxylamine hydrochloride; sodium hydrogencarbonate In water at 100℃; for 7h; Inert atmosphere; Sealed tube; Green chemistry; | A n/a B 87% |

-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| With zirconium(IV) chloride In nitromethane for 0.0333333h; Heating; | 86% |

| Conditions | Yield |
|---|---|
| With C22H48FeOP4 In tetrahydrofuran at 60℃; for 24h; Inert atmosphere; Schlenk technique; | A 86% B n/a |


| Conditions | Yield |
|---|---|
| With aluminum oxide; Oxone for 0.1h; microwave irradiation; | 84% |

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In acetonitrile at 20℃; for 0.75h; | A 84% B 10% |
| With 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In water; acetonitrile at 20℃; for 0.75h; | A 8% B 81% |

| Conditions | Yield |
|---|---|
| With sodium hydrogensulfite; sodium nitrite In ethanol; water; acetic acid at 24℃; for 4h; | 82% |

| Conditions | Yield |
|---|---|
| With [Pd{C6H4(CH2N(CH2Ph)2)}(μ-Br)]2; tetrabutylammomium bromide; potassium carbonate In N,N-dimethyl-formamide at 130℃; for 0.166667h; Microwave irradiation; chemoselective reaction; | 82% |
-
-
615-41-8
2-iodochlorobenzene

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| With [2,2]bipyridinyl; aluminum (III) chloride; nickel(II) acetylacetonate; zinc(II) oxide In 1,2-dimethoxyethane at 145℃; for 12h; | 81% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
156636-17-8
2-chlorobenzoselenoamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-Chlorobenzonitrile With woollins’ reagent In toluene for 4h; Heating; Stage #2: With water In toluene for 1h; | 100% |
| With selenium; sodium tetrahydroborate In ethanol for 8h; Heating; | 58% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
5720-05-8
4-methylphenylboronic acid

-
-
114772-53-1
2-Cyano-4'-methylbiphenyl

| Conditions | Yield |
|---|---|
| With potassium carbonate; dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate In n-heptane; water at 60℃; for 1.66667h; Suzuki Coupling; Inert atmosphere; | 100% |
| With potassium carbonate In water; isopropyl alcohol at 100℃; for 7h; Catalytic behavior; Suzuki-Miyaura Coupling; Inert atmosphere; | 100% |
| With bis({5-chloro-2-[(4-chlorophenyl)(hydroxyimino)methyl]phenyl})cyclodipalladachl-orane-1,3,4-tris(ylium)-2-uide; tetra(n-butyl)ammonium hydroxide; potassium carbonate; tri tert-butylphosphoniumtetrafluoroborate In N,N-dimethyl-formamide at 130℃; for 0.666667h; Suzuki-Miyaura coupling; Microwave irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; catacxium A; palladium diacetate In toluene at 100℃; for 20h; Suzuki reaction; | 100% |
| With potassium carbonate; 2-(di-tert-butyl-phosphino)-1-phenyl-1H-pyrrole; palladium diacetate In toluene at 60℃; Suzuki coupling; | 99% |
| With potassium phosphate; catacxium A; palladium diacetate In toluene at 100℃; for 20h; Suzuki cross-coupling reaction; | 99% |

| Conditions | Yield |
|---|---|
| With tris(2,6-di-tert-butylphenyl) phosphite; tetrabutyl-ammonium chloride; sodium carbonate; Palladacycle In N,N-dimethyl acetamide at 160℃; for 24h; Heck reaction; | 100% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); ruphos In tetrahydrofuran at 70℃; Negishi coupling; | 100% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
873-32-5
2-Chlorobenzonitrile

-
-
591-51-5
phenyllithium

-
-
17599-40-5
2-Chlor-N-trimethylsilyl-benzophenon-imin

| Conditions | Yield |
|---|---|
| Stage #1: 2-Chlorobenzonitrile; phenyllithium In tetrahydrofuran at -78℃; Inert atmosphere; Stage #2: chloro-trimethyl-silane In tetrahydrofuran at 20℃; Inert atmosphere; | 100% |

-
-
873-32-5
2-Chlorobenzonitrile

-
-
1647-23-0
3,3-dimethylbutyl bromide

-
-
1147270-29-8
1-(2-chlorophenyl)-4,4-dimethylpentan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 3,3-dimethylbutyl bromide With iodine; magnesium In tetrahydrofuran for 1.83333h; Reflux; Stage #2: 2-Chlorobenzonitrile With copper(l) iodide In tetrahydrofuran for 3.5h; Reflux; Stage #3: With hydrogenchloride In tetrahydrofuran; water at 20 - 50℃; for 1h; | 100% |
| Stage #1: 3,3-dimethylbutyl bromide With iodine; magnesium In tetrahydrofuran for 1.83333h; Reflux; Stage #2: 2-Chlorobenzonitrile With copper(l) iodide In tetrahydrofuran at 20℃; Reflux; | 100% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
1647-26-3
1-bromo-2-cyclohexylethane

-
-
1147270-20-9
1-(2-chlorophenyl)-3-cyclohexylpropan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-2-cyclohexylethane With iodine; magnesium In tetrahydrofuran for 1.83333h; Reflux; Stage #2: 2-Chlorobenzonitrile With copper(l) iodide In tetrahydrofuran for 3.5h; Reflux; Stage #3: With hydrogenchloride In tetrahydrofuran; water at 20 - 50℃; for 1h; | 100% |
| Stage #1: 1-bromo-2-cyclohexylethane With iodine; magnesium In tetrahydrofuran for 1.83333h; Reflux; Stage #2: 2-Chlorobenzonitrile With copper(l) iodide In tetrahydrofuran Reflux; | 100% |
-
-
873-32-5
2-Chlorobenzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 1-(2-bromo-ethyl)-adamantane With iodine; magnesium In tetrahydrofuran for 1.83333h; Reflux; Stage #2: 2-Chlorobenzonitrile With copper(l) iodide In tetrahydrofuran for 3.5h; Reflux; Stage #3: With hydrogenchloride In tetrahydrofuran; water at 50℃; for 1h; | 100% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
773-37-5
1-(2-bromo-ethyl)-adamantane

-
-
1147270-33-4
1-(2-chlorophenyl)-3-(1-adamantyl)propan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 1-(2-bromo-ethyl)-adamantane With iodine; magnesium In tetrahydrofuran for 1.83333h; Reflux; Stage #2: 2-Chlorobenzonitrile With copper(l) iodide In tetrahydrofuran at 20℃; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; silica gel for 0.0666667h; microwave irradiation; | 99% |
| With dihydrogen peroxide; sodium hydroxide In water at 35℃; for 10h; Large scale; | 98.5% |
| With water; potassium carbonate at 150℃; for 0.25h; Microwave irradiation; | 97% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
22261-94-5
N-(1-methyl-4-piperidinyl)aniline

-
-
93591-95-8
4-[2-(2-chlorobenzoyl)anilino]-1-methylpiperidine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; boron trichloride In water; toluene | 99% |
| With hydrogenchloride; boron trichloride 1) toluene, reflux, 1 h; 2) neat, 150 deg C, 3 h; 3) H2O, 100 deg C, 20 min; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; palladium diacetate; CyPF-t-Bu In 1,2-dimethoxyethane at 110℃; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide for 15h; Heating; | 98% |
| With fac-tris[2-phenylpyridinato-C2,N]iridium(III); caesium carbonate In N,N-dimethyl-formamide at 25℃; for 12h; Schlenk technique; Inert atmosphere; Irradiation; | 88% |
| With 1,2,3,4-tetrahydroquinolin-8-ol; caesium carbonate; copper(I) bromide In N,N-dimethyl-formamide at 130℃; for 48h; Inert atmosphere; chemoselective reaction; | 72% |
| With [2,2]bipyridinyl; (2,2'-bipyridine)nickel(II) dibromide In various solvent(s) Ambient temperature; electrolysis, magnesium anode, stainless steel cathode, 30 mA/cm2; | 50% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
60-23-1
Cysteamine

-
-
49672-22-2
2-(2-chlorophenyl)-4,5-dihydro-1,3-thiazole

| Conditions | Yield |
|---|---|
| With tribromomelamine at 100℃; for 0.0666667h; Neat (no solvent); | 99% |
| In ethanol for 5h; Heating; | 40% |
-
-
292638-84-7
styrene

-
-
873-32-5
2-Chlorobenzonitrile

-
-
80663-50-9, 38175-96-1
2-((1E)-2-phenylvinyl)benzenecarbonitrile

| Conditions | Yield |
|---|---|
| With sodium acetate; (IMes)Pd(NQ) In Bu4NBr at 140℃; for 24h; Conversion of starting material; Heck Reaction; | 99% |
| With tetrabutylammomium bromide; sodium acetate; C84H60N12O8Pd4 at 140℃; for 2h; Heck Reaction; Inert atmosphere; Schlenk technique; | 96% |
| With tris(2,6-di-tert-butylphenyl) phosphite; tetrabutyl-ammonium chloride; sodium carbonate; Palladacycle In N,N-dimethyl acetamide at 160℃; for 24h; Heck reaction; | 94% |

| Conditions | Yield |
|---|---|
| With ammonium formate In water at 20℃; for 6h; | 99% |
| With Triethoxysilane; C20H24N4Ni; sodium t-butanolate In toluene at 80℃; for 8h; Kumada Cross-Coupling; Inert atmosphere; Schlenk technique; | 83% |
| With cyclohexa-1,4-diene; 9-ethyl-N3,N3,N6,N6,-tetramethyl-9H-carbazole-3,6-diamine; N-ethyl-N,N-diisopropylamine In N,N-dimethyl acetamide at 23℃; for 0.8h; Inert atmosphere; UV-irradiation; Schlenk technique; | 83% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
16419-60-6
2-Methylphenylboronic acid

-
-
157366-46-6
2-(o-Tolyl)benzonitrile

| Conditions | Yield |
|---|---|
| With potassium fluoride; 18-crown-6 ether; bis-phenanthrylimidazolium chloride; palladium diacetate In tetrahydrofuran at 50℃; for 12h; Suzuki-Miyaura coupling; | 99% |
| With potassium phosphate; [2-(dicylohexylphopshino)-2',6'-dimethoxybiphenyl-Pd]2*2BF4 In toluene at 70℃; for 0.5h; Suzuki-Miyaura coupling; | 99% |
| With potassium phosphate; C28H31ClN2Ni; triphenylphosphine In tert-Amyl alcohol; toluene at 110℃; for 24h; Suzuki-Miyaura Coupling; Inert atmosphere; Sealed tube; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 12h; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 150℃; for 3h; Inert atmosphere; Green chemistry; | 97% |
| With potassium carbonate; copper(II) oxide In N,N-dimethyl-formamide at 120℃; for 12h; | 91% |

| Conditions | Yield |
|---|---|
| With sodium acetate; (IMes)Pd(NQ) In Bu4NBr at 140℃; for 24h; Conversion of starting material; Heck Reaction; | 99% |

| Conditions | Yield |
|---|---|
| With dichloro[1,1′-bis[bis(1,1-dimethylethyl)phosphino]ferrocene-P,P′]palladium; TPGS-750-M; triethylamine In water at 40℃; Inert atmosphere; | 99% |
| With potassium carbonate In ethanol; water at 120℃; for 2.5h; Suzuki-Miyaura reaction; continuous flow reactor; | 54% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
221626-03-5
2-cyclo-pentylbenzonitrile

| Conditions | Yield |
|---|---|
| With Pd-PEPPSI-IPrAn In tetrahydrofuran; 1,4-dioxane at 0 - 20℃; for 0.5h; Negishi Coupling; Schlenk technique; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In water; chlorobenzene at 60 - 65℃; for 17h; Inert atmosphere; | 99% |
| With tetrabutylammomium bromide In water; chlorobenzene at 70 - 75℃; for 15h; Inert atmosphere; | 98% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
17933-03-8
m-tolylboronic acid

| Conditions | Yield |
|---|---|
| With potassium phosphate tribasic trihydrate; C30H39P; palladium diacetate In tetrahydrofuran at 110℃; for 3h; Suzuki-Miyaura Coupling; Inert atmosphere; | 99% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
10365-98-7
3-methoxyphenylboronic acid

| Conditions | Yield |
|---|---|
| With C42H32N8O8Pd(2+)*2Cl(1-); tetrabutylammomium bromide; potassium carbonate In water at 100℃; for 1h; Suzuki-Miyaura Coupling; High pressure; | 99% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
873-74-5
4-Aminobenzonitrile

-
-
117847-16-2
2-((4-cyanophenyl)amino)benzonitrile

| Conditions | Yield |
|---|---|
| With sodium phenoxide; C47H70BrO4PPdSi In tetrahydrofuran at 20℃; for 1h; Schlenk technique; Inert atmosphere; Sealed tube; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Chlorobenzonitrile; benzaldehyde With hydrogen In 2-methyltetrahydrofuran at 100℃; under 11251.1 Torr; for 20h; High pressure; Autoclave; Stage #2: With hydrogenchloride In diethyl ether | 99% |
-
-
873-32-5
2-Chlorobenzonitrile

-
-
50907-46-5
5-(2-chlorophenyl)-1H-tetrazole

| Conditions | Yield |
|---|---|
| With sodium azide In N,N-dimethyl-formamide at 120℃; for 4h; | 98% |
| With sodium azide at 120℃; for 2h; | 97% |
| With sodium azide at 120℃; for 4.5h; | 97% |
2-Chlorobenzonitrile Chemical Properties
IUPAC Name: 2-Chlorobenzonitrile
Following is the structure of o-chlorotolunitrile (CAS NO.873-32-5):
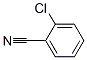
Molecular formula:C7H4ClN
Molecular Weight: 137.57
EINECS: 212-836-5
Index of Refraction: 1.563
Molar Refractivity: 36.14 cm3
Molar Volume: 111.2 cm3
Density: 1.23 g/cm3
Flash Point: 100.8 °C
Melting point: 43-46 °C(lit.)
Polarizability: 14.33 10-24cm3
Surface Tension: 46.2 dyne/cm
Enthalpy of Vaporization: 46.95 kJ/mol
Boiling Point: 232.8 °C at 760 mmHg
Vapour Pressure: 0.0577 mmHg at 25 °C
Appearance of o-chlorotolunitrile (CAS NO.873-32-5): white to light yellow crystal powder
Product Categories: Aromatic Nitriles; Chlorine Compounds; Nitriles; Alpha sort; C; CAlphabetic; CHEnvironmental Standards; Metabolites; Pesticides & Metabolites; C6 to C7; Cyanides/Nitriles; Nitrogen Compounds
Canonical SMILES: C1=CC=C(C(=C1)C#N)Cl
InChI: InChI=1S/C7H4ClN/c8-7-4-2-1-3-6(7)5-9/h1-4H
InChIKey: NHWQMJMIYICNBP-UHFFFAOYSA-N
2-Chlorobenzonitrile Toxicity Data With Reference
| 1. | eye-rbt 100 mg/24H MOD | 28ZPAK Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku Marhold, J.V.,Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha,Czechoslovakia.: 1972,160. | ||
| 2. | orl-mus LD50:>300 mg/kg | JMCMAR Journal of Medicinal Chemistry. 21 (1978),906. | ||
| 3. | ipr-mus LD50:150 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD691-490 . |
o-chlorotolunitrile (CAS NO.873-32-5) hasn't been listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65. And the toxicological properties have not been fully investigated. You can see actual entry in RTECS for complete information.
2-Chlorobenzonitrile Consensus Reports
Reported in EPA TSCA Inventory. Cyanide and its compounds are on the Community Right-To-Know List.
2-Chlorobenzonitrile Safety Profile
Poison by intraperitoneal route. Moderately toxic by ingestion. An eye irritant. When heated to decomposition or on contact with water, steam, acid, or acid fumes it emits toxic fumes of Cl− and CN−. See also NITRILES.
Hazard Codes:  Xn,
Xn, Xi
Xi
Hazard Note:Harmful/Irritant
The Risk Statements information of o-chlorotolunitrile (CAS NO.873-32-5): 21/22-36-21/22/23
36: Irritating to the eyes
21/22: Harmful in contact with skin and if swallowed
20/21/22: Harmful by inhalation, in contact with skin and if swallowed
The Safety Statements information of o-chlorotolunitrile (CAS NO.873-32-5): 23
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
RIDADR:3439
WGK Germany:2
RTECS:DI2625000
2-Chlorobenzonitrile Specification
o-chlorotolunitrile , its cas register number 873-32-5. It also can be called NSC 8438 ; Nitril kyseliny o-chlorbenzoove ; Nitril kyseliny o-chlorbenzoove [Czech] ; o-Chlorbenzonitril ; o-Chlorbenzonitril [Czech] ; o-Chlorobenzonitrile ; o-Chlorocyanobenzene ; and o-Cyanochlorobenzene . Its classification code is Skin / Eye Irritant.
o-chlorotolunitrile (CAS NO.873-32-5) could be stable under normal temperatures and pressures. It should avoid the condition like incompatible materials. It is not compatible with strong oxidizing agents, strong reducing agents, strong acids, strong bases. And also prevent it to broken down into hazardous decomposition products: hydrogen chloride, nitrogen oxides, carbon monoxide, carbon dioxide. However, its hazardous polymerization has not been reported.
Related Products
- 2-Chlorobenzonitrile
- 87333-19-5
- 87333-22-0
- 873-38-1
- 873397-34-3
- 873-41-6
- 873417-25-5
- 873-42-7
- 873429-58-4
- 873429-59-5
- 87343-53-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View