-
Name
2-Picoline
- EINECS 203-643-7
- CAS No. 109-06-8
- Article Data276
- CAS DataBase
- Density 0.94 g/cm3
- Solubility miscible with water
- Melting Point -70 °C(lit.)
- Formula C6H7N
- Boiling Point 127.544 °C at 760 mmHg
- Molecular Weight 93.1283
- Flash Point 26.111 °C
- Transport Information UN 2313 3/PG 3
- Appearance colourless to yellow liquid with an unpleasant smell
- Safety 26-36
- Risk Codes 10-20/21/22-36/37
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms RCRA waste number U191;CCRIS 1721;AI3-24109;AI3-2409;alpha-Methylpyridine;o-Picoline;
- PSA 12.89000
- LogP 1.39000
Synthetic route

-
-
187737-37-7
propene

-
A
-
109-06-8
α-picoline

| Conditions | Yield |
|---|---|
| <10 min, 23°C, 1 atm.; monitored by NMR; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| With benzyl alcohol at 120℃; for 6h; Inert atmosphere; | 99% |
| With cis-Cyclooctene; trans-dioxo(5,10,15,20-tetramesitylporphirinato)ruthenium(VI) In benzene at 80℃; for 15h; | 96% |
| With diphosphorus tetraiodide In dichloromethane Heating; 10-20 min; | 95% |

| Conditions | Yield |
|---|---|
| at 150℃; for 22h; Autoclave; | 94.3% |
| With decaethylene glycol mono n-hexadecyl ether; cyclopentadienyl-cyclooctadienyl-cobalt(I) In water for 4h; Ambient temperature; Irradiation; | 18% |
| (η5-cyclopentadienyl)-η4-cycloocta-1,5-dienecobalt(I) at 40℃; for 2h; Irradiation; Yield given; | |
| trimethylsilyl modified Cp-CO catalyst at 130 - 152℃; under 15001.2 Torr; for 2h; | |
| η5-cyclopentadienylbis(ethene)cobalt at 40℃; for 3h; Product distribution; in the dark; variation of reaction time, temperature, catalyst. The influence of light and irradiation was investigated.; |

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; cesium fluoride In 2-pentanol at 100℃; for 18h; Inert atmosphere; | A 93.1% B 6.9% |
-
-
110-86-1
pyridine

-
A
-
110-89-4
piperidine

-
B
-
109-06-8
α-picoline

-
C
-
24362-44-5
1,5-di-(1-piperidyl)pentane

| Conditions | Yield |
|---|---|
| With hydrogen; Ni-Cr catalyst at 160℃; under 30400 Torr; for 8h; Product distribution; temperatures from 140 to 192 deg C, pressure 20 to 65 atm, reaction time 5 - 8 h; | A 91.8% B n/a C 0.1% |


| Conditions | Yield |
|---|---|
| With Fe-MnOx-Yb at 475℃; Gas phase; | 89.5% |
| nickel(II) nitrate for 8h; Ambient temperature; Irradiation; | 37.1% |
| With nano-diatomite modified magnesium aluminum hydrotalcite at 140 - 500℃; Temperature; |

| Conditions | Yield |
|---|---|
| With samarium diiodide; phosphoric acid In tetrahydrofuran for 0.000555556h; Ambient temperature; | 88% |
-
-
126963-92-6
benzyltri(2-(6-methylpyridyl))phosphonium bromide

-
A
-
109-06-8
α-picoline

-
B
-
4411-80-7
6,6'-dimethyl-2,2'-bipyridine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 10h; Heating; | A 68% B 87% C n/a |
| With hydrogenchloride In water for 10h; Product distribution; Heating; variation of pH, temp. and time; | A 68% B 87% C n/a |
| With hydrogenchloride In water for 10h; Heating; | A 68% B 68% C n/a |

-
-
14402-20-1
N-cetyl-α-methylpyridinium iodide

-
-
109-06-8
α-picoline

| Conditions | Yield |
|---|---|
| With triethylammonium hydrogensulfite at 150℃; for 30h; Mechanism; | 86% |
-
-
38700-15-1
triphenyl-(2-pyridylmethyl)phosphonium chloride

-
-
109-06-8
α-picoline

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; benzyl alcohol In tetrahydrofuran at 40℃; for 6h; Inert atmosphere; | 80% |
-
-
13115-43-0
2-pyridineacetic acid

-
-
109-06-8
α-picoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In chloroform at 20℃; for 24h; Irradiation; Inert atmosphere; | 79% |

| Conditions | Yield |
|---|---|
| With Raney nickel at 180℃; under 51716.2 Torr; for 0.5h; Concentration; Flow reactor; Green chemistry; regioselective reaction; | 78% |

| Conditions | Yield |
|---|---|
| With Ra-Ni for 212h; Reflux; regioselective reaction; | 71% |

-
-
109-06-8
α-picoline

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); sodium isopropylate; 1,3-bis[(2,6-diisopropyl)phenyl]imidazolinium chloride In 2-methyltetrahydrofuran at 80℃; for 12h; Green chemistry; | 71% |
| With bis(tricyclohexylphosphine)nickel(II) dichloride; potassium iodide; zinc In 1,4-dioxane; methanol at 60℃; for 20h; Inert atmosphere; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-iodopyridine; bis(iodozinc)methane With triphenylphosphine; nickel dichloride In tetrahydrofuran at 40℃; Stage #2: With hydrogenchloride In tetrahydrofuran; water Reagent/catalyst; chemoselective reaction; | 71% |

| Conditions | Yield |
|---|---|
| With hydrogen In para-xylene at 120℃; under 750.075 Torr; for 24h; Glovebox; Sealed tube; chemoselective reaction; | 71% |

| Conditions | Yield |
|---|---|
| In perchloric acid addn. of Na2CO3;; | A 70% B 30% |
| In perchloric acid |

| Conditions | Yield |
|---|---|
| With hydrogen; GIPKh-105 copper-chromium catalyst at 200℃; Product distribution; Mechanism; other isomeric pyridinecarboxaldehydes; var. temp.; | A 14% B 66% |
-
-
4377-33-7
2-chloromethylpyridine

-
-
100-52-7
benzaldehyde

-
A
-
109-06-8
α-picoline

-
B
-
2294-74-8
1-phenyl-2-pyridin-2-yl-ethanol

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; silver nitrate; zinc In water at 30℃; for 1h; | A 51% B 66% |


| Conditions | Yield |
|---|---|
| With [IrCl(CO)(PPh3)2]; hydrazine hydrate; potassium hydroxide In methanol at 160℃; for 3h; Wolff-Kishner Reduction; Sealed tube; | 62% |
| With [IrCl(CO)(PPh3)2]; hydrazine hydrate; potassium hydroxide In methanol at 160℃; for 3h; Sealed tube; | 62% |
| With hydrazine hydrate; C23H40MnNO2P2; potassium tert-butylate In tert-butyl alcohol at 115℃; for 48h; Wolff-Kishner Reduction; Green chemistry; | 26% |

| Conditions | Yield |
|---|---|
| With ammonia; Pb(5.3percent)/borotitano silicate at 420℃; for 1h; Product distribution / selectivity; Molecular sieve; | A 2.4% B 61.3% C 21.6% |
| With ammonia; Pb/SnS-1B at 395℃; Product distribution / selectivity; | A 1% B 53.4% C 22.3% |
| With ammonia; titanium-silicate catalyst (sample C) at 250 - 400℃; Conversion of starting material; | A 0.88% B 53.44% C 20.55% |

-
-
50-00-0
formaldehyd

-
-
75-07-0
acetaldehyde

-
A
-
110-86-1
pyridine

-
B
-
109-06-8
α-picoline

-
C
-
108-89-4
picoline

-
D
-
108-99-6
3-Methylpyridine

| Conditions | Yield |
|---|---|
| With Pb-ZSM-5 zeolite; ammonia In gas | A 60% B 7% C 4% D 8% |
| With Co-ZSM-5 zeolite; ammonia In gas | A 57% B 6% C 8% D 7% |
| With Ag-ZSM-5 zeolite; ammonia In gas | A 42% B 3% C 6% D 11% |
| With pentasil zeolite H-ZSM-5; ammonia In gas at 450℃; Product distribution; synthesis of pyridine bases over ion-exchanged pentasil zeolite; var. zeolites, var. Si/Al atomic ratio; | A 42% B 3% C 5% D 11% |

-
-
1452-77-3
pyridine-2-carboxylic acid amide

-
A
-
109-06-8
α-picoline

-
B
-
109-05-7, 3000-79-1
2-Methylpiperidin

| Conditions | Yield |
|---|---|
| With hydrogenchloride; samarium for 0.166667h; Ambient temperature; | A 13% B 60% |

| Conditions | Yield |
|---|---|
| With n-butyllithium; 2-(N,N-dimethylamino)ethanol 1.) hexane, -78 deg C, 1 h, 2.) THF, -78 deg C, 1 h; | A 60% B 16 % Chromat. |

-
-
67-64-1
acetone

-
-
74-86-2
acetylene

-
A
-
109-06-8
α-picoline

-
B
-
108-48-5
2,6-dimethylpyridine

-
C
-
108-75-8
2,4,6-trimethyl-pyridine

-
D
-
108-47-4
2,4-lutidine

| Conditions | Yield |
|---|---|
| With ammonia; MG-4 at 375℃; under 760 Torr; | A 6.3% B 7.3% C 58.3% D 5.9% |
| With ammonia; MG-4 at 375℃; under 760 Torr; Product distribution; other catalysts; | A 6.3% B 7.3% C 58.3% D 5.9% |
| With ammonia; MG-4 at 350℃; under 760 Torr; | A 6.2% B 15.6% C 36.4% D 6.8% |


| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 40℃; for 6h; | A 58% B 26% |

-
-
3678-63-5
4-chIoro-2-methylpyridine

-
-
73183-34-3
bis(pinacol)diborane

-
A
-
109-06-8
α-picoline

-
B
-
660867-80-1
2-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine

| Conditions | Yield |
|---|---|
| With bis(1,3-dimesityl-1H-imidazol-2(3H)-ylidene)nickel(0); potassium methanolate In hexane at 25℃; for 6h; Inert atmosphere; Irradiation; | A 10 %Chromat. B 55% |

-
-
931-19-1
2-methylpyridine N-oxide

-
-
16420-13-6
N,N-Dimethylthiocarbamoyl chloride

-
A
-
109-06-8
α-picoline

| Conditions | Yield |
|---|---|
| In acetonitrile at 81℃; for 4h; | A 52% B 3% C 5% |


| Conditions | Yield |
|---|---|
| With water-d2 In water-d2 | A 50% B 50% |
| With D2O In water-d2 |

| Conditions | Yield |
|---|---|
| In toluene at 110℃; for 19h; | 100% |
| In acetone for 8h; Heating; | 51% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; Na12[WZn3(H2O)2(ZnW9O34)2] at 75℃; for 7h; | 100% |
| With 2,2,2-Trifluoroacetophenone; dihydrogen peroxide; acetonitrile In tert-butyl alcohol at 20℃; for 18h; Green chemistry; chemoselective reaction; | 99% |
| With dihydrogen peroxide In acetic acid at 70 - 75℃; for 24h; Oxidation; | 98% |

| Conditions | Yield |
|---|---|
| 100% | |
| In acetone Reflux; | 100% |
| In toluene for 14h; Inert atmosphere; Reflux; | 72% |

| Conditions | Yield |
|---|---|
| With phenyllithium In diethyl ether for 2.5h; Heating; | 100% |
-
-
109-06-8
α-picoline

-
-
565-80-0
2,4-dimethylpentan-3-one

-
-
941279-81-8
2,4-dimethyl-3-(pyridin-2-ylmethyl)pentan-3-ol

| Conditions | Yield |
|---|---|
| Stage #1: α-picoline With tert.-butyl lithium In tetrahydrofuran; pentane at -30℃; Stage #2: 2,4-dimethylpentan-3-one In tetrahydrofuran; pentane at -30 - 20℃; Stage #3: With water In tetrahydrofuran; pentane | 100% |
| Stage #1: α-picoline With n-butyllithium In tetrahydrofuran; hexane at -78 - -50℃; for 1h; Inert atmosphere; Stage #2: 2,4-dimethylpentan-3-one In tetrahydrofuran; hexane at -50℃; for 2h; Inert atmosphere; | 91% |
| Stage #1: α-picoline With n-butyllithium In tetrahydrofuran; hexane at -78 - -50℃; for 1h; Inert atmosphere; Stage #2: 2,4-dimethylpentan-3-one In tetrahydrofuran; hexane at -50℃; for 2h; Inert atmosphere; Stage #3: With water In tetrahydrofuran; hexane Inert atmosphere; | 90% |
-
-
109-06-8
α-picoline

-
-
345298-30-8
di-μ-chloro-dichloro-bis[η5-(perfluorobutyl)tetramethylcyclopentadienyl]-dirhodium(III)

-
-
933802-63-2
dichloro-(perfluorohexyl)tetramethylcyclopentadienyl-(2-methylpyridine)-rhodium(III)

| Conditions | Yield |
|---|---|
| In chloroform (Ar); methylpyridine was added to soln. of Rh complex in CHCl3; mixt. was stirred for 2 h at room temp.; evapd.; dried (vac.); | 100% |
-
-
109-06-8
α-picoline

-
-
119-60-8
dicyclohexyl ketone

-
-
102658-00-4
1,1-dicyclohexyl-2-(pyridin-2-yl)ethanol

| Conditions | Yield |
|---|---|
| Stage #1: α-picoline With n-butyllithium In tetrahydrofuran; hexane at -78 - -20℃; Inert atmosphere; Stage #2: dicyclohexyl ketone In tetrahydrofuran; hexane at -20℃; Inert atmosphere; Stage #3: With water; ammonium chloride In tetrahydrofuran; hexane | 100% |
-
-
109-06-8
α-picoline

-
-
1765-40-8
(bromomethyl)pentafluorobenzene

-
-
1229616-85-6
N-(pentafluorobenzyl)-2-methylpyridinium bromide

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 24h; | 100% |
-
-
109-06-8
α-picoline

-
-
83766-52-3
4-heptadecafluorooctylaniline

| Conditions | Yield |
|---|---|
| With sodiumsulfide nonahydrate; sulfur for 72h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 101℃; for 16h; Inert atmosphere; | 100% |
-
-
109-06-8
α-picoline

| Conditions | Yield |
|---|---|
| Stage #1: α-picoline With potassium tert-butylate at -35℃; for 0.333333h; Stage #2: With n-butyllithium In hexane at -35 - 20℃; for 4h; | 100% |
| With n-butyllithium; potassium tert-butylate In hexane at 20℃; for 1h; Schlenk technique; Glovebox; | 63% |
-
-
109-06-8
α-picoline

| Conditions | Yield |
|---|---|
| for 0.0833333h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; for 0.166667h; Glovebox; Inert atmosphere; | 100% |


| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 24h; | 100% |
-
-
109-06-8
α-picoline

-
-
2632-13-5
2-Bromo-4'-methoxyacetophenone

-
-
7496-82-4
2-(4-methoxyphenyl)-indolizine

| Conditions | Yield |
|---|---|
| Stage #1: α-picoline; 2-Bromo-4'-methoxyacetophenone In acetone for 1h; Heating; Stage #2: With potassium carbonate for 5h; | 99% |
-
-
109-06-8
α-picoline

-
-
15743-13-2
tetrachloro-cyclopent-4-ene-1,3-dione

| Conditions | Yield |
|---|---|
| In formic acid at 100℃; Product distribution; CH3CO2H,CF3CO2H; | 99% |
| With acetic acid Ambient temperature; | 87.4% |
-
-
109-06-8
α-picoline

-
-
105-36-2
ethyl bromoacetate

-
-
55814-02-3
1-(2-ethoxy-2-oxoethyl)-2-methylpyridin-1-ium bromide

| Conditions | Yield |
|---|---|
| In ethanol at 60 - 80℃; for 20h; | 99% |
| In tetrahydrofuran at 80℃; for 12h; Inert atmosphere; | 92% |
| for 16h; Reflux; | 90% |
-
-
109-06-8
α-picoline

-
-
26127-08-2
(1R,2S,5R)-1-(chloromethoxy)-2-isopropyl-5-methylcyclohexane

| Conditions | Yield |
|---|---|
| In hexane at 20℃; Menschutkin quaternization; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: α-picoline With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -5℃; for 0.5h; Stage #2: phenanthrene-3-carbaldehyde In tetrahydrofuran; hexane at 20℃; for 0.5h; Further stages.; | 99% |
-
-
109-06-8
α-picoline

-
-
68558-86-1
thallium(I) pentafluorobenzoate

-
-
41575-66-0
(SP-4-3)-dichlorido(N,N-dimethyl-ethane-1,2-diamine)platinum(II)

-
-
363-72-4
Pentafluorobenzene

-
-
117533-75-2
b-chloro-c(N),d(N')-{N,N-dimethyl-N'-(2,3,5,6-tetrafluorophenyl)ethane-1,2-diaminato(1-)}-a-(2-methylpyridine)platinum(II)

-
B
-
124-38-9
carbon dioxide

| Conditions | Yield |
|---|---|
| In further solvent(s) Heating under stirring (110-115°C, N2, 120 min, 2-methylpyridine).; Cooling, evapn. to dryness (vacuum), washed (ether, petrol), dried, extraction with acetone, filtn. of TlCl, evapn. to dryness, washed (cold ethanol), elem. anal.; | A 28% B 99% |

-
-
109-06-8
α-picoline

| Conditions | Yield |
|---|---|
| In neat (no solvent) standing in vapor of amine (room temp., 2 days); | 99% |
| In benzene addn. of amine to Fe-complex; distn. off of excess amine and solvent (after 24 h), drying (vac., room temp.); |
-
-
109-06-8
α-picoline

-
-
202280-80-6, 1046824-89-8
trans-[Pd(AsPh3)2(3,5-dichlorotrifluorophenyl)2]

-
-
202280-69-1
cis-[Pd(2-picoline)2(3,5-dichlorotrifluorophenyl)2]

| Conditions | Yield |
|---|---|
| In dichloromethane excess of picoline, stirring for 30 min; evapn., washing (hexane), drying; elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| In dichloromethane excess of picoline, stirring for 30 min; evapn., washing (hexane), drying; elem. anal.; | 99% |
-
-
109-06-8
α-picoline

-
-
185226-19-1, 70424-04-3
trans-chloromethylbis(dimethylsulfoxide)platinum(II)

-
-
185042-99-3
cis(C,N)-chloromethyl(dimethyl sulfoxide)(2-methylpyridine)platinum(II)

| Conditions | Yield |
|---|---|
| In dichloromethane stirring stoich. amts. for 30 min; addn. of pentane, pptn. on cooling, collection (filtration), washing (cold pentane), drying (vac.); elem. anal.; | 99% |
-
-
109-06-8
α-picoline

-
-
623-03-0
4-Cyanochlorobenzene

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
1202924-71-7
3-(4-chlorophenyl)pyrido[1,2-c]pyrimidin-1-one

| Conditions | Yield |
|---|---|
| Stage #1: α-picoline With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -70℃; for 1h; Inert atmosphere; Stage #2: 4-Cyanochlorobenzene In tetrahydrofuran; hexane at -70 - 20℃; Inert atmosphere; Stage #3: bis(trichloromethyl) carbonate With triethylamine In tetrahydrofuran; hexane at 20℃; for 2h; Inert atmosphere; | 99% |


| Conditions | Yield |
|---|---|
| at 20℃; for 504h; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With C50H63NO2Zr; trityl tetrakis(pentafluorophenyl)borate In chlorobenzene at 100℃; for 6h; Inert atmosphere; Glovebox; | 99% |
| With tris(pentafluorophenyl)borate; C42H51N3PSc In chlorobenzene at 100℃; for 15h; Inert atmosphere; Schlenk technique; Glovebox; | 82% |
| With C23H29N2Sc; triphenylcarbenium tetra(pentafluorophenyl)borate at 70℃; Schlenk technique; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With C50H63NO2Zr; trityl tetrakis(pentafluorophenyl)borate In chlorobenzene at 100℃; for 6h; Inert atmosphere; Glovebox; | 99% |
| With tris(pentafluorophenyl)borate; C42H51N3PSc In chlorobenzene at 100℃; for 15h; Inert atmosphere; Schlenk technique; Glovebox; | 81% |
| With C23H29N2Sc; triphenylcarbenium tetra(pentafluorophenyl)borate at 70℃; Schlenk technique; Inert atmosphere; |
2-Picoline Specification
The 2-Methylpyridine, or 2-Picoline, is the compound described with formula C6H7N. With the CAS registry number 109-06-8, it is a colorless liquid. This chemical's classification codes are Mutation Data; Skin / Eye Irritant. Its EINECS registry number is 203-643-7. In addition, its IUPAC name is called 2-methylpyridine.
Physical properties of 2-Methylpyridine: (1)ACD/LogP: 1.22; (2)ACD/LogD (pH 5.5): 0.64; (3)ACD/LogD (pH 7.4): 1.204; (4)ACD/BCF (pH 5.5): 1.31; (5)ACD/BCF (pH 7.4): 4.798; (6)ACD/KOC (pH 5.5): 28.898; (7)ACD/KOC (pH 7.4): 105.888; (8)#H bond acceptors: 1; (9)Index of Refraction: 1.501; (10)Molar Refractivity: 29.169 cm3; (11)Molar Volume: 98.932 cm3; (12)Surface Tension: 34.098 dyne/cm; (13)Density: 0.941 g/cm3; (14)Flash Point: 26.111 °C; (15)Enthalpy of Vaporization: 36.17 kJ/mol; (16)Boiling Point: 127.544 °C at 760 mmHg; (17)Vapour Pressure: 13.468 mmHg at 25°C.
Preparation: 2-Methylpyridine was the first pyridine compound reported to be isolated in pure form. It is now mainly produced by two principal routes, the condensation of acetaldehyde, formaldehyde, and ammonia and the cyclization of nitriles and acetylene. One example of such reaction is the combination of acetaldehyde and ammonia:
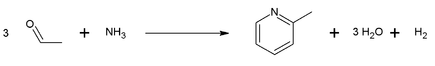
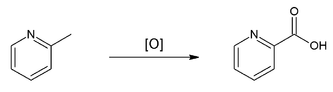
Uses of 2-Methylpyridine: it is a versatile building block and can be used as a precursor to a variety of derivatives. For example, oxidation by potassium permanganate affords picolinic acid:
When you are using this chemical, please be cautious about it as the following:
This chemical may cause damage to health. It is harmful by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing.
You can still convert the following datas into molecular structure:
(1)SMILES: Cc1ccccn1
(2)InChI: InChI=1/C6H7N/c1-6-4-2-3-5-7-6/h2-5H,1H3
(3)InChIKey: BSKHPKMHTQYZBB-UHFFFAOYAR
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | oral | 900mg/kg (900mg/kg) | Hygiene and Sanitation Vol. 33(10-12), Pg. 341, 1968. | |
| mouse | LC50 | inhalation | 9gm/m3 (9000mg/m3) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 57(9-10), Pg. 64, 1992. | |
| mouse | LD50 | intraperitoneal | 529mg/kg (529mg/kg) | Toxicon. Vol. 23, Pg. 815, 1985. | |
| mouse | LD50 | oral | 674mg/kg (674mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: EXCITEMENT | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 45(12), Pg. 62, 1980. |
| rabbit | LD50 | skin | 410uL/kg (0.41mL/kg) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 4, Pg. 119, 1951. | |
| rat | LCLo | inhalation | 4000ppm/4H (4000ppm) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 4, Pg. 119, 1951. | |
| rat | LD50 | intraperitoneal | 200mg/kg (200mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Fundamental and Applied Toxicology. Vol. 5, Pg. 920, 1985. |
| rat | LD50 | oral | 790mg/kg (790mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: EXCITEMENT | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 45(12), |
Related Products
- 2-Picoline
- 2-Picoline-3-boronic acid HCl
- 2-Picoline-N-oxide
- 109069-75-2
- 109073-77-0
- 109074-67-1
- 1090-78-4
- 109-07-9
- 109-08-0
- 109086-16-0
- 109086-17-1
- 109089-77-2
- 109-09-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View