-
Name
3-Bromobiphenyl
- EINECS 218-304-9
- CAS No. 2113-57-7
- Article Data89
- CAS DataBase
- Density 1.363 g/cm3
- Solubility insoluble in water
- Melting Point 9oC
- Formula C12H9Br
- Boiling Point 300 °C at 760 mmHg
- Molecular Weight 233.107
- Flash Point 135.6 °C
- Transport Information
- Appearance Colorless to pale yellow liquid
- Safety 37/39-26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Biphenyl,3-bromo- (6CI,7CI,8CI);1-Bromo-3-phenylbenzene;3-Biphenyl bromide;3-Bromo-1,1'-biphenyl;3-Xenyl bromide;NSC 407775;PBB 2;[1,1'-Biphenyl]-3-yl bromide;m-Bromobiphenyl;
- PSA 0.00000
- LogP 4.11610
Synthetic route

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate; triphenylphosphine In water; toluene at 100℃; for 24h; | 98% |
| With barium dihydroxide; tetrakis(triphenylphosphine) palladium(0) In 1,4-dioxane; water for 6h; Suzuki coupling; Heating; | 92% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In 1,2-dimethoxyethane; water for 8h; Inert atmosphere; Reflux; | 80% |

| Conditions | Yield |
|---|---|
| With C27H23ClNO3PPd; potassium carbonate In ethanol; water at 50℃; for 7h; Temperature; Suzuki-Miyaura Coupling; Schlenk technique; | 94% |
| With palladium diacetate In ethanol at 20℃; for 0.25h; Suzuki-Miyaura Coupling; chemoselective reaction; | 86% |
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; toluene at 90℃; for 3h; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: benzene With potassium permanganate; acetic acid Microwave irradiation; Stage #2: (3-bromophenyl)boronic acid for 0.5h; Microwave irradiation; | 91% |
| With potassium permanganate; acetic acid Heating; | 90% |
| With manganese triacetate for 0.5h; Heating; | 89% |
| With iron(III) trifluoromethanesulfonate; di-tert-butyl peroxide; 4,7-bis[4-(trifluoromethyl)phenyl]-1,10-phenanthroline at 80℃; for 24h; | 76% |
| With NH-pyrazole; potassium phosphate; iron(III) sulphate heptahydrate; 1,4,7,10-tetraazacyclododecan at 80℃; for 48h; | 55% |

| Conditions | Yield |
|---|---|
| With potassium permanganate; acetic acid Heating; | 90% |
| With manganese triacetate Heating; | 73% |
-
-
66107-31-1
3-bromophenyl trifluoromethanesulfonate

-
-
38111-44-3
phenylzinc(II) bromide

-
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| bis(diphenylphosphino)propanepalladium(II) dichloride In tetrahydrofuran at 60℃; for 11h; Negishi cross-coupling reaction; | 90% |

-
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With 1% Pd/C In methanol at 50℃; for 0.5h; Suzuki-Miyaura reaction; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| at 100℃; for 12h; Sealed tube; | 89% |
| With meta-dinitrobenzene |

| Conditions | Yield |
|---|---|
| Stage #1: bromobenzene; 1-Bromo-3-iodobenzene With magnesium In tetrahydrofuran for 1h; Inert atmosphere; Reflux; Green chemistry; Stage #2: In tetrahydrofuran at 50 - 85℃; for 8h; Green chemistry; | 84.69% |

| Conditions | Yield |
|---|---|
| With diethyl bromomethylmalonate; [4,4′-di-tert-butyl-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C}iridium(III) hexafluorophosphate; caesium carbonate at 80℃; for 22h; Irradiation; | 82% |
| With dipotassium peroxodisulfate; silver nitrate In acetonitrile at 120℃; for 24h; Glovebox; Schlenk technique; Sealed tube; Inert atmosphere; | 55% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver nitrate In acetonitrile at 120℃; for 10h; Inert atmosphere; Sealed tube; Green chemistry; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-Bromo-3-iodobenzene; chlorobenzene With magnesium In tetrahydrofuran for 1h; Inert atmosphere; Reflux; Green chemistry; Stage #2: In tetrahydrofuran; water; toluene at 50 - 85℃; for 8h; Inert atmosphere; Reflux; Green chemistry; | 81.37% |

| Conditions | Yield |
|---|---|
| Stage #1: iodobenzene; 1-Bromo-3-iodobenzene With magnesium In tetrahydrofuran for 1h; Inert atmosphere; Reflux; Green chemistry; Stage #2: In tetrahydrofuran at 50 - 85℃; for 8h; Green chemistry; | 80.61% |

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver nitrate In acetonitrile at 120℃; for 20h; Sealed tube; Schlenk technique; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With potassium phosphate In 1,4-dioxane at 115℃; for 30h; Suzuki-Miyaura Coupling; Inert atmosphere; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dibromobenzene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.5h; Inert atmosphere; Stage #2: C19H16NS(1+)*F6P(1-) In tetrahydrofuran; hexane at -78 - 23℃; for 1.5h; Inert atmosphere; | 71% |

| Conditions | Yield |
|---|---|
| 70% |
-
-
108-36-1
1,3-dibromobenzene

-
-
98-80-6
phenylboronic acid

-
A
-
92-06-8
1,3-diphenylbenzene

-
B
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With disodium[(N,N’-bis(2-hydroxy-5-sulfonatobenzyl)-1,2-diphenyl-1,2-diaminoethano)palladate(II)]; caesium carbonate In water at 80℃; for 1h; Suzuki-Miyaura Coupling; | A 68% B 24% |
| With potassium phosphate; {1,3-di(NHP(piperidinyl)2)C6H3}PdCl In toluene at 100℃; for 0.0833333h; Suzuki reaction; | A 76 % Chromat. B 24 % Chromat. |
| With potassium carbonate; [{P(NC4H8NMe)3}2PdCl2] In methanol at 20℃; for 2h; Suzuki-Miyaura coupling reaction; |


| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; potassium 2-methylbutan-2-olate at 80℃; for 24h; Inert atmosphere; | 65% |
-
-
66107-31-1
3-bromophenyl trifluoromethanesulfonate

-
A
-
92-06-8
1,3-diphenylbenzene

-
B
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With lithium bromide; bis(diphenylphosphino)propanepalladium(II) dichloride In diethyl ether at 0℃; for 2h; Kumada cross-coupling reaction; | A 20% B 60% |


| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; 1,2-Dinitrobenzene In decane at 150℃; for 12h; Inert atmosphere; Sealed tube; | 59% |
-
-
108-86-1
bromobenzene

-
-
108-95-2
phenol

-
A
-
2052-07-5
2-Bromobiphenyl

-
B
-
92-66-0
4-bromo-1,1'-biphenyl

-
C
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; iodine at 289.9℃; for 0.0272222h; Product distribution; | A 22.7% B 18.4% C 58.9% |


| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; 1,2-Dinitrobenzene In decane at 150℃; for 12h; Inert atmosphere; Sealed tube; | 56% |
-
-
591-18-4
1-Bromo-3-iodobenzene

-
-
71-43-2
benzene

-
A
-
92-06-8
1,3-diphenylbenzene

-
B
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With 1-(2-hydroxyethyl)piperazine; potassium tert-butylate at 100℃; for 24h; Sealed tube; Inert atmosphere; | A 50% B 25% |


| Conditions | Yield |
|---|---|
| With (triphenylphosphine)gold(I) chloride In acetonitrile at 20℃; for 16h; Inert atmosphere; | 44% |
-
-
19920-85-5
1-(3-bromophenyl)cyclohexanol

-
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With copper; Selectfluor In acetonitrile at 80℃; for 24h; chemoselective reaction; | 41% |
-
-
108-36-1
1,3-dibromobenzene

-
-
71-43-2
benzene

-
A
-
92-06-8
1,3-diphenylbenzene

-
B
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; vasicine at 20 - 110℃; for 72h; Schlenk technique; Inert atmosphere; | A 24% B 19% |

-
-
108-86-1
bromobenzene

-
-
17420-03-0
N-sulphinylphenylhydrazine

-
A
-
2052-07-5
2-Bromobiphenyl

-
B
-
92-66-0
4-bromo-1,1'-biphenyl

-
C
-
2113-57-7
3-bromobiphenyl

-
D
-
882-33-7
diphenyldisulfane

| Conditions | Yield |
|---|---|
| at 135℃; for 72h; Further byproducts given; | A 20.9% B 6.9% C 12.7% D 1.1% E n/a |
| at 135℃; for 72h; Further byproducts given; | A 19.5% B 8.1% C 12.9% D 1.1% E n/a |

-
-
108-86-1
bromobenzene

-
-
17420-03-0
N-sulphinylphenylhydrazine

-
A
-
1212-08-4
S-Phenyl benzenethiosulfonate

-
B
-
2052-07-5
2-Bromobiphenyl

-
C
-
92-66-0
4-bromo-1,1'-biphenyl

-
D
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| at 135℃; for 72h; Further byproducts given; | A 0.2% B 20.9% C 6.9% D 12.7% E n/a |

-
-
108-86-1
bromobenzene

-
-
17420-03-0
N-sulphinylphenylhydrazine

-
A
-
2052-07-5
2-Bromobiphenyl

-
B
-
92-66-0
4-bromo-1,1'-biphenyl

-
C
-
139-66-2
diphenyl sulfide

-
D
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| at 135℃; for 72h; Further byproducts given; | A 20.9% B 6.9% C 0.8% D 12.7% E n/a |

-
-
110-86-1
pyridine

-
-
108-86-1
bromobenzene

-
-
94-36-0
dibenzoyl peroxide

-
A
-
1008-89-5
2-phenylpyridine

-
B
-
2052-07-5
2-Bromobiphenyl

-
C
-
92-66-0
4-bromo-1,1'-biphenyl

-
D
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| at 70℃; |

-
-
50448-95-8
2-ethylhexyl 3-sulfanylpropanoate

-
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); N-ethyl-N,N-diisopropylamine; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 110℃; for 18h; Inert atmosphere; | 100% |
-
-
206357-82-6
(3-bromophenyl)(pyridin-2-yl)methanone

-
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromobiphenyl With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.666667h; Stage #2: (3-bromophenyl)(pyridin-2-yl)methanone In tetrahydrofuran; hexane at -78 - 20℃; for 16h; | 99% |

| Conditions | Yield |
|---|---|
| With tris(1-adamantyl)phosphine; palladium diacetate; lithium hexamethyldisilazane In toluene at 70℃; for 0.55h; Inert atmosphere; | 99% |
-
-
2113-57-7
3-bromobiphenyl

-
-
13331-27-6
m-nitrobenzene boronic acid

-
-
78626-53-6
1‐(3‐nitrophenyl)‐3‐phenylbenzene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium hydrogencarbonate In 1,2-dimethoxyethane; water for 16h; Suzuki Coupling; Inert atmosphere; Reflux; | 98% |
-
-
6223-78-5
2,5-dichloro-2,5-dimethyl hexane

-
-
2113-57-7
3-bromobiphenyl

-
-
288259-76-7
6-(3-bromophenyl)-1,2,3,4-tetrahydro-1,1,4,4-tetramethylnaphthalene

| Conditions | Yield |
|---|---|
| With aluminium trichloride In 1,2-dichloro-ethane at 0℃; Condensation; | 97% |
-
-
2113-57-7
3-bromobiphenyl

-
-
10365-98-7
3-methoxyphenylboronic acid

-
-
156941-81-0
3-methoxy-[1,1';3',1'']terphenyl

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); sodium carbonate In ethanol; toluene for 3h; Suzuki coupling; Heating; | 97% |
-
-
2113-57-7
3-bromobiphenyl

-
-
100-53-8
phenylmethanethiol

-
-
94306-39-5
[1,1'-biphenyl]-3-yl(benzyl)sulfane

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); N-ethyl-N,N-diisopropylamine; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; Inert atmosphere; Sealed tube; | 97% |
| With tris-(dibenzylideneacetone)dipalladium(0); N-ethyl-N,N-diisopropylamine; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; Inert atmosphere; Sealed tube; | 97% |
-
-
2113-57-7
3-bromobiphenyl

-
-
1346669-48-4
9-(4-biphenylyl)-3,3'-bicarbazole

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromobiphenyl; 9-(4-biphenylyl)-3,3'-bicarbazole With potassium tert-butylate In toluene for 0.5h; Inert atmosphere; Stage #2: With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; XPhos In toluene for 10h; Reflux; | 97% |

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate In toluene for 2h; Inert atmosphere; Reflux; | 95% |
| With tri-tert-butyl phosphine; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate In toluene at 100℃; for 24h; | 85% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium carbonate; N,N`-dimethylethylenediamine In toluene for 120h; Reflux; | 95% |

| Conditions | Yield |
|---|---|
| With C43H59NO2PPd(1+)*CH3O3S(1-); caesium carbonate In toluene at 80℃; Schlenk technique; Inert atmosphere; | 95% |
-
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; palladium diacetate; sodium t-butanolate In toluene at 110℃; for 12h; | 95% |

-
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| Stage #1: (R)-2,2'-(2,2'-bis(methoxymethoxy)-1,1'-binaphthyl-3,3'-diyl)bis(4,4',5,5'-tetramethyl-1,3,2-dioxaborolane); 3-bromobiphenyl With tetrakis(triphenylphosphine) palladium(0) In 1,4-dioxane; water at 120℃; Alkaline conditions; Stage #2: With hydrogenchloride In 1,4-dioxane; methanol; dichloromethane; water at 80℃; | 95% |

| Conditions | Yield |
|---|---|
| With piperidine; copper(l) iodide; tetrakis(triphenylphosphine) palladium(0) at 80℃; for 5h; Hagihara coupling; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromobiphenyl With n-butyllithium In tetrahydrofuran; hexanes at -78℃; Inert atmosphere; Stage #2: 2-(diisopropylsilyl)pyridine In tetrahydrofuran; hexanes at -78 - -30℃; Inert atmosphere; | 93% |
| Stage #1: 3-bromobiphenyl With n-butyllithium In tetrahydrofuran; hexanes at -78℃; Inert atmosphere; Stage #2: 2-(diisopropylsilyl)pyridine In tetrahydrofuran; hexanes at -78 - 30℃; Inert atmosphere; | 93% |
-
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With water-d2; potassium carbonate; silver carbonate; cyclohexyldiphenylphosphine In toluene at 120℃; for 12h; | 93% |
-
-
2113-57-7
3-bromobiphenyl

-
-
20442-79-9
3-iodo-1,1'-biphenyl

| Conditions | Yield |
|---|---|
| With copper(l) iodide; cis-N,N'-dimethyl-1,2-diaminocyclohexane; sodium iodide In 1,4-dioxane Reflux; Inert atmosphere; | 92% |
| With iodine; sodium iodide In acetonitrile at 20℃; for 36h; Inert atmosphere; UV-irradiation; Green chemistry; | 87% |
-
-
2113-57-7
3-bromobiphenyl

-
-
16523-54-9
chlorodicyclohexylphosphane

-
-
640735-28-0
[1,1'-biphenyl]-3-yldicyclohexylphosphane

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromobiphenyl With n-butyllithium In diethyl ether; hexane at -20℃; for 2h; Inert atmosphere; Stage #2: chlorodicyclohexylphosphane In diethyl ether; hexane at 20℃; for 6h; Cooling; | 92% |
-
-
2113-57-7
3-bromobiphenyl

-
-
123088-32-4
4-tert-butyl-dimethylsilyloxyphenylboronic acid anhydride

-
-
139265-73-9
[1,1’:3’,1’’-terphenyl]-4-ol

| Conditions | Yield |
|---|---|
| With sodium carbonate; tetrakis(triphenylphosphine) palladium(0) In 1,2-dimethoxyethane; water at 95℃; Suzuki reaction; | 91% |

| Conditions | Yield |
|---|---|
| With methanol; magnesium at 20℃; | 91% |
| With 2-H-1,3-di-tert-butyl-1,3,2-diazaphosphorinane; 2,2'-azobis(isobutyronitrile) In toluene at 90℃; for 5h; | 83% |
| With sodium formate In water; isopropyl alcohol at 50℃; for 0.00222222h; microflow reactor; | 99 %Chromat. |
-
-
2113-57-7
3-bromobiphenyl

-
-
99685-96-8, 161105-99-3, 161106-00-9, 111138-12-6, 133318-63-5, 134053-11-5, 134931-35-4, 134931-36-5, 139703-76-7, 145633-27-8, 175414-73-0, 175414-74-1, 175414-75-2, 175519-12-7, 175519-13-8, 175519-14-9, 175519-15-0, 136376-46-0, 144906-37-6, 144906-38-7, 151767-00-9, 152882-97-8, 152882-98-9, 152882-99-0, 153062-34-1, 154171-74-1, 154171-75-2, 154333-99-0, 154334-00-6, 154397-63-4, 154460-59-0, 199456-56-9, 108739-25-9, 120329-57-9, 120329-58-0
fullerene-C60

| Conditions | Yield |
|---|---|
| Stage #1: 3-bromobiphenyl With iodine; magnesium In tetrahydrofuran at 20℃; for 2.5h; Inert atmosphere; Reflux; Stage #2: With copper(I) bromide dimethylsulfide complex In tetrahydrofuran at -78℃; for 0.333333h; Inert atmosphere; Stage #3: fullerene-C60 In tetrahydrofuran; 1,2-dichloro-benzene at -78 - 20℃; Inert atmosphere; | 91% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; trans-1,2-cyclohexanediamine In 1,4-dioxane at 120℃; for 18h; | 90% |
-
-
108714-73-4
9,9-dimethyl-9H-fluoren-2-ylamine

-
-
2113-57-7
3-bromobiphenyl

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos In toluene at 108℃; for 4h; Buchwald-Hartwig Coupling; Inert atmosphere; | 90% |
| With tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate; XPhos In toluene at 108℃; for 4h; Inert atmosphere; | 74.94% |
3-Bromobiphenyl Consensus Reports
Reported in EPA TSCA Inventory.
3-Bromobiphenyl Specification
The 3-Bromobiphenyl, with the CAS registry number 2113-57-7 and EINECS registry number 218-304-9, is also called 1,1'-Biphenyl, 3-bromo-. It is a kind of colorless to pale yellow liquid, and belongs to the following product categories: Biphenyl derivatives; Biphenyl & Diphenyl ether; Aryl; C9 to C12; Halogenated Hydrocarbons. And the molecular formula of this chemical is C12H9Br.
The physical properties of 3-Bromobiphenyl are as following: (1)ACD/LogP: 4.65; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 4.65; (4)ACD/LogD (pH 7.4): 4.65; (5)ACD/BCF (pH 5.5): 2026.88; (6)ACD/BCF (pH 7.4): 2026.88; (7)ACD/KOC (pH 5.5): 8102.6; (8)ACD/KOC (pH 7.4): 8102.6; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 0 Å2; (13)Index of Refraction: 1.6; (14)Molar Refractivity: 58.53 cm3; (15)Molar Volume: 170.9 cm3; (16)Polarizability: 23.2×10-24cm3; (17)Surface Tension: 40.4 dyne/cm; (18)Density: 1.363 g/cm3; (19)Flash Point: 135.6 °C; (20)Enthalpy of Vaporization: 51.84 kJ/mol; (21)Boiling Point: 300 °C at 760 mmHg; (22)Vapour Pressure: 0.00205 mmHg at 25°C.
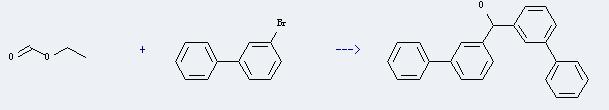
Uses of 3-Bromobiphenyl: It can react with formic acid ethyl ester to produce 3,3'-diphenylbenzhydrol. This reaction will need reagent Mg, and the solvent diethyl ether. The reaction time is 18 hours with heating, and the yield is about 41%.
You should be cautious while dealing with this chemical. It irritates eyes, respiratory system and skin. Therefore, you had better take the following instructions: Wear suitable protective clothing, gloves and eye/face protection, and in case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: Brc2cc(c1ccccc1)ccc2
(2)InChI: InChI=1/C12H9Br/c13-12-8-4-7-11(9-12)10-5-2-1-3-6-10/h1-9H
(3)InChIKey: USYQKCQEVBFJRP-UHFFFAOYAD
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | intraperitoneal | 500mg/kg (500mg/kg) | "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 6, Pg. 217, 1954. |
Related Products
- 3-Bromobiphenyl
- 2113-58-8
- 211364-78-2
- 211366-30-2
- 21136-70-9
- 2113-68-0
- 21138-24-9
- 21139-31-1
- 21139-32-2
- 2114-00-3
- 2114-02-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View