-
Name
4-tert-Amylphenol
- EINECS 201-280-9
- CAS No. 80-46-6
- Article Data59
- CAS DataBase
- Density 0.96 g/cm3
- Solubility 37 mg/L (20 ºC) in water
- Melting Point 88-89 °C(lit.)
- Formula C11H16O
- Boiling Point 262.499 °C at 760 mmHg
- Molecular Weight 164.247
- Flash Point 122.379 °C
- Transport Information UN 2430 8/PG 2
- Appearance White little spiculate crystal
- Safety 26-27-36/37/39-45-61
- Risk Codes 21/22-34-51/53
-
Molecular Structure
-
Hazard Symbols
 C,
C, N
N
- Synonyms Phenol,p-(1,1-dimethylpropyl)- (5CI);Phenol, p-tert-pentyl- (6CI,8CI);4-(1,1-Dimethylpropyl)phenol;4-t-Pentylphenol;4-tert-Pentylphenol;Amilfenol;p-(1,1-Dimethylpropyl)phenol;p-(a,a-Dimethylpropyl)phenol;p-tert-Amylphenol;p-tert-Pentylphenol;
- PSA 20.23000
- LogP 3.07980
Synthetic route
-
-
261618-80-8
1-allyloxy-4-(1',1''-dimethylpropyl)benzene

-
-
80-46-6
4-t-amylphenol

| Conditions | Yield |
|---|---|
| With tert.-butyl lithium In pentane at -78 - 20℃; Substitution; | 94% |
| Stage #1: 1-allyloxy-4-(1',1''-dimethylpropyl)benzene With C12H37NiP4(1+)*C2F6NO4S2(1-) In tetrahydrofuran at 20℃; for 0.5h; Glovebox; Schlenk technique; Inert atmosphere; Stage #2: With toluene-4-sulfonic acid In tetrahydrofuran for 15h; Glovebox; Schlenk technique; Reflux; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In chloroform at 25℃; for 10h; | 82% |
| With toluene-4-sulfonic acid at 100℃; im Einschlussrohr; | |
| With metal halides |
-
-
513-35-9
2-methyl-but-2-ene

-
-
115-11-7
isobutene

-
-
108-95-2
phenol

-
A
-
98-54-4
para-tert-butylphenol

-
B
-
88-18-6
2-tert-Butylphenol

-
C
-
80-46-6
4-t-amylphenol

-
D
-
96-76-4
2,4-di-tert-Butylphenol

-
E
-
732-26-3
2,4,6-tri-tert-butylphenoxol

-
F
-
120-95-6
2,4-di-tert-amylphenol

-
G
-
122269-03-8
2-t-butyl-4-(1,1-dimethylpropyl)-phenol

-
H
-
122269-05-0
4-tert-butyl-2-(1,1-dimethyl-propyl)-phenol

| Conditions | Yield |
|---|---|
| Stage #1: isobutene; phenol; Fulcat 22B catalyst at 130 - 140℃; for 1.5h; Inert atmosphere; Stage #2: 2-methyl-but-2-ene at 130℃; for 3.25h; | A 50.8% B 1.4% C 15.3% D 17.6% E 0.3% F 1.3% G 10.7% H 10.7% |


| Conditions | Yield |
|---|---|
| at 75 - 180℃; |

| Conditions | Yield |
|---|---|
| at 100℃; |


| Conditions | Yield |
|---|---|
| With sulfuric acid | |
| With metal halides | |
| With diphenyl hydrogen phosphate | |
| With metal halides | |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With iron(III) chloride |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; Petroleum ether | |
| With sulfuric acid | |
| With sulfuric acid; zinc(II) chloride |

| Conditions | Yield |
|---|---|
| With zinc(II) chloride at 180℃; | |
| With zinc(II) chloride at 180℃; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride |

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 35 - 110℃; | |
| With sulfuric acid at 170℃; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 99.9 - 209.9℃; Equilibrium constant; effect of temperature; |

| Conditions | Yield |
|---|---|
| zuletzt bei 50-60grad; |

-
-
7705-08-0
iron(III) chloride

-
-
628-50-2
carbonochloridic acid 3-methyl-butyl ester

-
-
108-95-2
phenol

-
-
80-46-6
4-t-amylphenol


| Conditions | Yield |
|---|---|
| at 25 - 30℃; |


| Conditions | Yield |
|---|---|
| at 50 - 60℃; |


| Conditions | Yield |
|---|---|
| zuletzt bei 50-60grad; |


| Conditions | Yield |
|---|---|
| With aluminium trichloride at 50 - 60℃; |

-
-
80-46-6
4-t-amylphenol

| Conditions | Yield |
|---|---|
| Diazotization; |

| Conditions | Yield |
|---|---|
| With aluminium trichloride at 110 - 120℃; |

-
-
7446-70-0
aluminium trichloride

-
-
120-95-6
2,4-di-tert-amylphenol

-
-
108-95-2
phenol

-
-
80-46-6
4-t-amylphenol

| Conditions | Yield |
|---|---|
| at 110℃; |


| Conditions | Yield |
|---|---|
| at 170℃; |


| Conditions | Yield |
|---|---|
| die Diazoverbindung reagiert; |
-
-
86840-38-2
benzyl-(4-tert-pentyl-phenyl)-ether

-
-
80-46-6
4-t-amylphenol

| Conditions | Yield |
|---|---|
| With palladium diacetate; cyclohexene at 60℃; Hydrogenolysis; | |
| With trimethylsilyl trifluoromethanesulfonate; methyl-phenyl-thioether In dichloromethane; trifluoroacetic acid at 20℃; for 0.0833333h; dealkylation; |

| Conditions | Yield |
|---|---|
| With sulfated zirconia at 140℃; for 1h; |

-
-
513-35-9
2-methyl-but-2-ene

-
-
108-95-2
phenol

-
A
-
80-46-6
4-t-amylphenol

-
B
-
120-95-6
2,4-di-tert-amylphenol

| Conditions | Yield |
|---|---|
| Dowex DR 2030 at 80℃; for 7h; Inert atmosphere; |

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
80-46-6
4-t-amylphenol

-
-
851231-25-9
[4-(1,1-dimethylpropyl)phenyl]trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 0.5h; Inert atmosphere; | 99.7% |
| With pyridine In dichloromethane at 0℃; for 0.25h; | 98% |
| With pyridine at 20℃; for 3h; Cooling with ice; | 43.5 g |
| With pyridine at 20℃; for 3h; Cooling with ice; | 43.5 g |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 22h; | 99% |
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 22h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 7h; | 97% |
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 7h; | 97% |
-
-
80-46-6
4-t-amylphenol

-
-
49608-01-7
6-chloro-3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 80℃; for 96h; | 96% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 1h; | 94% |
-
-
4549-32-0
1,8-dibromooctane

-
-
80-46-6
4-t-amylphenol

-
-
94441-79-9
1-((8-bromooctyl)oxy)-4-(tert-pentyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: 4-t-amylphenol With sodium n-propoxide In propan-1-ol at 20℃; for 0.25h; Stage #2: 1,8-dibromooctane In propan-1-ol at 60℃; for 6h; Reflux; | 93% |
| With sodium carbonate In water Heating; |
-
-
80-46-6
4-t-amylphenol

-
-
35787-71-4
thianthrene cation radical perchlorate

| Conditions | Yield |
|---|---|
| In acetonitrile for 0.5h; | 93% |

| Conditions | Yield |
|---|---|
| A n/a B 92.6% |

-
-
80-46-6
4-t-amylphenol

-
-
527737-18-4
1-(2,2,3,3-tetrafluoro-propoxy)-3-(4-tert-amylphenoxy)-2-propanol

| Conditions | Yield |
|---|---|
| 92% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 74h; Reflux; | 92% |
-
-
80-46-6
4-t-amylphenol

-
-
106-95-6
allyl bromide

-
-
261618-80-8
1-allyloxy-4-(1',1''-dimethylpropyl)benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 91% |
| With potassium carbonate; acetone |
-
-
80-46-6
4-t-amylphenol

-
-
75-65-0
tert-butyl alcohol

-
-
122269-03-8
2-t-butyl-4-(1,1-dimethylpropyl)-phenol

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic acid at 80℃; for 36h; Inert atmosphere; | 90% |
| With sodium hydroxide In trifluoroacetic acid |
-
-
80-46-6
4-t-amylphenol

-
-
26254-35-3
5-bromoacenaphthylene-1,2-dione

-
-
1265828-91-8
5-(4-tert-amylphenoxy)acenaphthenequinone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 4h; | 88% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 4h; | 85% |

| Conditions | Yield |
|---|---|
| In methanol; water for 6h; Mannich reaction; Heating; | 86% |

-
-
80-46-6
4-t-amylphenol

-
-
20698-30-0
trans-4-tert-amylcyclohexano-1-ol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; Raney Ni-Al; water at 90℃; for 12h; | 83.5% |
| With hydrogen; nickel In ethanol at 128 - 140℃; under 98800 - 114000 Torr; |

| Conditions | Yield |
|---|---|
| montmorillonite acid clay for 0.0333333h; microwave irradiation; | 83% |
| With sodium acetate |
-
-
80-46-6
4-t-amylphenol

-
-
350-46-9
4-Fluoronitrobenzene

-
-
61405-51-4
1-nitro-4-(4-(tert-pentyl)phenoxy)benzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 2h; | 83% |
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 2h; | 83% |
| With potassium hydroxide at 150 - 160℃; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 80 - 120℃; for 72h; | 83% |
-
-
4549-31-9
1,7-dibromoheptane

-
-
80-46-6
4-t-amylphenol

-
-
94254-07-6
1-((7-bromoheptyl)oxy)-4-(tert-pentyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: 4-t-amylphenol With sodium n-propoxide In propan-1-ol at 20℃; for 0.25h; Stage #2: 1,7-dibromoheptane In propan-1-ol at 60℃; for 6h; Reflux; | 82% |
| With sodium carbonate In water Heating; |
-
-
80-46-6
4-t-amylphenol

-
-
109-64-8
1,3-dibromo-propane

-
-
53606-50-1
1-(3-bromopropoxy)-4-(tert-pentyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: 4-t-amylphenol With sodium proprionate at 20℃; for 0.0833333h; Stage #2: 1,3-dibromo-propane at 60℃; for 7h; | 82% |
| With sodium carbonate In water Heating; | |
| With sodium n-propoxide at 60℃; for 6h; Reflux; |
-
-
80-46-6
4-t-amylphenol

-
-
87728-50-5
methyl propyl p-tert-amylphenyl phosphate

| Conditions | Yield |
|---|---|
| With sodium In benzene Heating; | 82% |
-
-
50-00-0
formaldehyd

-
-
80-46-6
4-t-amylphenol

-
-
93503-77-6
5,11,17,23,29,35,41,47-octakis(1,1-dimethylpropyl)-49,50,51,52,53,54,55,56-octahydroxycalix<8>arene

| Conditions | Yield |
|---|---|
| With tetramethyl ammoniumhydroxide In diphenylether; 5,5-dimethyl-1,3-cyclohexadiene; water for 12h; Reagent/catalyst; Solvent; Inert atmosphere; Reflux; | 81.2% |
-
-
111-24-0
1,5-dibromo-pentane

-
-
80-46-6
4-t-amylphenol

-
-
93144-54-8
1-((5-bromopentyl)oxy)-4-(tert-pentyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: 4-t-amylphenol With sodium n-propoxide In propan-1-ol at 20℃; for 0.25h; Stage #2: 1,5-dibromo-pentane In propan-1-ol at 60℃; for 6h; Reflux; | 80% |
| Stage #1: 4-t-amylphenol With sodium n-propoxide In propan-1-ol at 20℃; for 0.0833333h; Stage #2: 1,5-dibromo-pentane In propan-1-ol at 60℃; for 7h; Reflux; | 77% |
| With sodium carbonate In water Heating; |
-
-
80-46-6
4-t-amylphenol

| Conditions | Yield |
|---|---|
| With N,N'-di-tert-butylethylenediamine; oxygen; copper(II) acetate monohydrate In dichloromethane at 25℃; under 1520.1 Torr; for 4h; Catalytic behavior; Glovebox; | 80% |
-
-
110-52-1
1,4-dibromo-butane

-
-
80-46-6
4-t-amylphenol

-
-
92731-14-1
1-((4-bromobutyl)oxy)-4-(tert-pentyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: 4-t-amylphenol With sodium n-propoxide In propan-1-ol at 20℃; for 0.25h; Stage #2: 1,4-dibromo-butane In propan-1-ol at 60℃; for 6h; Reflux; | 78% |
| Stage #1: 4-t-amylphenol With sodium proprionate at 20℃; for 0.0833333h; Stage #2: 1,4-dibromo-butane at 60℃; for 7h; | 78% |
| With sodium carbonate In water Heating; |
-
-
629-03-8
1 ,6-dibromohexane

-
-
80-46-6
4-t-amylphenol

-
-
20011-21-6
1-((6-bromohexyl)oxy)-4-(tert-pentyl)benzene

| Conditions | Yield |
|---|---|
| Stage #1: 4-t-amylphenol With sodium n-propoxide In propan-1-ol at 20℃; for 0.25h; Stage #2: 1 ,6-dibromohexane In propan-1-ol at 60℃; for 6h; Reflux; | 76% |
| Stage #1: 4-t-amylphenol With sodium n-propoxide In propan-1-ol at 20℃; for 0.0833333h; Stage #2: 1 ,6-dibromohexane In propan-1-ol at 60℃; for 7h; Reflux; | 59% |
| With sodium carbonate In water Heating; |
-
-
80-46-6
4-t-amylphenol

-
-
16420-13-6
N,N-Dimethylthiocarbamoyl chloride

-
-
928794-11-0
4-(1,1-dimethyl-propyl)-1-dimethylthiocarbamoyloxy-benzene

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 20℃; for 16h; | 76% |
-
-
80-46-6
4-t-amylphenol

-
-
485-47-2
indan-1,2,3-trione hydrate

-
-
1416230-72-2
4b,9b-dihydroxy-8-tert-pentyl-4-bH-benzo[d]indeno[1,2-b]furan-10(9bH)-one

| Conditions | Yield |
|---|---|
| With acetic acid for 32h; Reflux; | 70% |
| With acetic acid for 32h; Reflux; | 70% |
4-tert-Amylphenol Consensus Reports
Reported in EPA TSCA Inventory.
4-tert-Amylphenol Specification
The 4-tert-Amylphenol, with the CAS registry number 80-46-6, is also known as 1-Hydroxy-4(2-methyl-2-butyl)benzene. It belongs to the product categories of Industrial/Fine Chemicals; Alcohol& Phenol& Ethers; Alkylphenols (Environmental Endocrine Disruptors); Analytical Chemistry; Environmental Endocrine Disruptors; Organic Building Blocks; Oxygen Compounds; Phenols. Its EINECS registry number is 201-280-9. This chemical's molecular formula is C11H16O and molecular weight is 164.24. What's more, both its IUPAC name and systematic name are the same which is called 4-(2-Methylbutan-2-yl)phenol. It should be stored in a cool, dry place. This chemical can be used in organic synthesis.
Physical properties about 4-tert-Amylphenol are: (1)ACD/LogP: 3.702; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.70; (4)ACD/LogD (pH 7.4): 3.70; (5)ACD/BCF (pH 5.5): 383.03; (6)ACD/BCF (pH 7.4): 382.48; (7)ACD/KOC (pH 5.5): 2458.56; (8)ACD/KOC (pH 7.4): 2455.03; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 20.23 Å2; (13)Index of Refraction: 1.509; (14)Molar Refractivity: 51.154 cm3; (15)Molar Volume: 171.058 cm3; (16)Polarizability: 20.279×10-24cm3; (17)Surface Tension: 33.040 dyne/cm; (18)Density: 0.96 g/cm3; (19)Flash Point: 122.379 °C; (20)Enthalpy of Vaporization: 52.047 kJ/mol; (21)Boiling Point: 262.499 °C at 760 mmHg; (22)Vapour Pressure: 0.0070 mmHg at 25 °C.
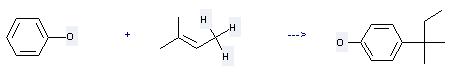
Preparation of 4-tert-Amylphenol: this chemical can be prepared by phenol with 2-methyl-but-2-ene. This reaction needs reagent AlCl3 and solvent CHCl3 at temperature of 25 °C. The reaction time is 10 hours. The yield is 82 %.
Uses of 4-tert-Amylphenol: (1) it is used to synthetic intermediates of organic mercury fungicide, pesticide and rubber; (2) it is used to produce other chemicals. For example, it can react with trichloromethyl-benzene to get [5-(1,1-dimethyl-propyl)-2-hydroxy-phenyl]-phenyl-methanone. This reaction needs reagent NaOH and solvent H2O at temperature of 75-80 °C. The reaction time is 2.5 hours. The yield is 43 %.
![4-tert-Amylphenol can react with trichloromethyl-benzene to get [5-(1,1-dimethyl-propyl)-2-hydroxy-phenyl]-phenyl-methanone.](/UserFilesUpload/Uses of 4-tert-Amylphenol.jpg)
When you are dealing with this chemical, you should be very careful. This chemical may destroy living tissue on contact and may present an immediate or delayed danger to one or more components of the environment. It is harmful in contact with skin and if swallowed. It may cause burns. In addition, this chemical is toxic to aquatic organisms and may cause long-term adverse effects in the aquatic environment. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. You must take off immediately all contaminated clothing and avoid releasing to the environment. In case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice. And in case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
(1) SMILES: Oc1ccc(cc1)C(C)(C)CC
(2) InChI: InChI=1S/C11H16O/c1-4-11(2,3)9-5-7-10(12)8-6-9/h5-8,12H,4H2,1-3H3
(3) InChIKey: NRZWYNLTFLDQQX-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rabbit | LD50 | skin | 2gm/kg (2000mg/kg) | Union Carbide Data Sheet. Vol. 8/13/1964, | |
| rat | LD50 | oral | 1830mg/kg (1830mg/kg) | Industrial Hygiene Foundation of America, Chemical and Toxicological Series, Bulletin. Vol. 6, Pg. 1, 1967. |
Related Products
- 4-tert-Amylphenol
- 80466-56-4
- 80466-76-8
- 80466-79-1
- 80466-80-4
- 8047-15-2
- 80471-63-2
- 8047-24-3
- 80473-92-3
- 80474-14-2
- 80474-45-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View