-
Name
Trimethylsilyl trifluoromethanesulfonate
- EINECS 248-565-4
- CAS No. 27607-77-8
- Article Data47
- CAS DataBase
- Density 1.276 g/cm3
- Solubility reacts with water
- Melting Point 25°C
- Formula C4H9F3O3SSi
- Boiling Point 140 °C at 760 mmHg
- Molecular Weight 222.26
- Flash Point 38.5 °C
- Transport Information UN 2920 8/PG 2
- Appearance clear colourless to light brown fuming liquid
- Safety 16-26-36/37/39-45-8
- Risk Codes 10-14-34
-
Molecular Structure
-
Hazard Symbols
 C,
C,  F
F
- Synonyms Methanesulfonicacid, trifluoro-, trimethylsilyl ester (8CI,9CI);Silanol, trimethyl-, trifluoromethanesulfonate(8CI);Trifluoromethanesulfonic acid trimethylsilyl ester;Trimethylsilanoltrifluoromethanesulfonate;Trimethylsilyl triflate;Trimethylsilyl trifluoromethylsulfonate;Trimethylsilyl trifluoromethanesulfonate;
- PSA 51.75000
- LogP 2.76830
Synthetic route
-
-
75-76-3
tetramethylsilane

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| for 1h; Ambient temperature; | 99% |
-
-
43112-38-5
3-(trimethylsilyl)-2-oxazolidinone

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| at 40℃; for 0.25h; | 98% |
| for 0.0333333h; | 80% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In n-heptane at 50 - 60℃; for 13h; Large scale; | 96.55% |
| for 7h; Heating; Yield given; | |
| at 20℃; | |
| at 40℃; for 24h; Schlenk technique; Inert atmosphere; |
-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
18156-74-6
1-(Trimethylsilyl)imidazole

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In diethyl ether at 10℃; for 1h; | 96% |
-
-
99542-57-1
(dimethylsilyl)methyl trifluoromethanesulfonate

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| at 80℃; for 9h; | 95% |
| In benzene-d6 at 135℃; Rate constant; Mechanism; τ1/2; | |
| In neat (no solvent) at 134.9℃; Rate constant; Thermodynamic data; lgA, EA, ΔH(excit.), ΔS(excit.), ΔG(excit.), ΔrH; | |
| at 80℃; Kinetics; Rate constant; |
-
-
18306-29-1
bis(trimethylsilyl)sulphate

-
-
2794-60-7
barium trifluoromethanesulfonate

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| at 140 - 150℃; | 95% |

-
-
2923-28-6
silver trifluoromethanesulfonate

-
A
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In benzene-d6 Inert atmosphere; | A 90% B 92% |

-
-
1066-40-6
Trimethylsilanol

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| Stage #1: Trimethylsilanol; trifluoromethylsulfonic anhydride at 45℃; for 3.5h; Stage #2: With chloro-trimethyl-silane at 15℃; for 2.5h; Temperature; | 89% |
-
-
107-46-0
Hexamethyldisiloxane

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| at 70 - 75℃; for 2h; | 88% |
-
-
5936-98-1
trichloromethyltrimethylsilane

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| at 120℃; for 0.833333h; | 88% |
-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
762-72-1
allyl-trimethyl-silane

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1h; | 85% |
| In dichloromethane at 0℃; for 2h; |
-
-
1066-40-6
Trimethylsilanol

-
-
421-83-0
trifluoromethane sulfonyl chloride

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| at 15℃; for 4h; Temperature; | 80% |
-
-
18163-07-0
2-(trimethylsilyl)propene

-
-
1493-13-6
trifluorormethanesulfonic acid

-
A
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
B
-
69739-34-0
t-butyldimethylsiyl triflate

| Conditions | Yield |
|---|---|
| In pentane at 0℃; for 0.166667h; Yield given; | A n/a B 66% |
| In pentane at 0℃; for 0.166667h; Yields of byproduct given; | A n/a B 66% |

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
81290-20-2
(trifluoromethyl)trimethylsilane

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With boron tribromide at -25 - 20℃; | 54% |
-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
10416-59-8, 132255-83-5
(Z)-trimethylsilyl N-trimethylsilylacetimidate

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| for 0.0833333h; | 43% |
-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
25436-07-1
trimethylsilyl trichloroacetate

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| for 0.0833333h; | 40% |
-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| for 0.0833333h; | 35% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
65597-24-2
Chlorine(I) trifluoromethanesulfonate

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| at -111 - 0℃; |
-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
97730-10-4
methyl trimethylsilyl tert-butylphosphonate

-
A
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
B
-
97730-01-3
O-methyl-tert-butylphosphonic trifluoromethanesulfonic anhydride

| Conditions | Yield |
|---|---|
| In dichloromethane for 12h; Ambient temperature; |

-
-
50732-21-3, 65428-75-3
N,N,N'-tris(trimethylsilyl)phosphenimidous amide

-
-
114684-87-6
bis(diisopropylamino)phosphanylium trifluoromethanesulfonate

-
A
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
B
-
65160-85-2, 66435-33-4
N,N,N',N'-tetraisopropyl-1,3-bis(trimethylsilanyl)[1,3,2,4]diazadiphosphetidine-2,4-diamine

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

-
-
50732-21-3, 65428-75-3
N,N,N'-tris(trimethylsilyl)phosphenimidous amide

-
A
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
B
-
66435-31-2, 66435-32-3, 344867-26-1
N,N,N',N'-Tetraethyl-1,3-bis-trimethylsilanyl-[1,3,2,4]diazadiphosphetidine-2,4-diamine

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

-
-
88016-29-9
μ-oxobis[(trifluoromethanesulfonato)(phenyl)iodine]

-
-
84140-30-7
4,4-dimethyl-1-(trimethylsilyl)-2-pentyne

-
A
-
26981-77-1
t-butylallene

-
B
-
107-46-0
Hexamethyldisiloxane

-
C
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
D
-
131251-47-3
4,4-dimethyl-1-(2-iodophenyl)-2-pentyne

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 at -80℃; Mechanism; or tributylstannyl compound; |

-
-
16393-88-7
trimethyl(trimethylstannyl)silane

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In dichloromethane at -78℃; |

-
A
-
420-56-4
trimethylsilyl fluoride

-
B
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With cesium fluoride In N,N,N,N,N,N-hexamethylphosphoric triamide |


-
A
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
B
-
97730-00-2
tert-butylphenylphosphinic trifluoromethanesulfonic anhydride

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; |
-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
2170-06-1
1-Phenyl-2-(trimethylsilyl)acetylene

-
A
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
B
-
100-41-4
ethylbenzene

| Conditions | Yield |
|---|---|
| With HW(CO)3(C5H5) In dichloromethane-d2 at 22℃; for 0.833333h; Product distribution; other times; without Cp(CO)3WH; | A 91 % Spectr. B 85 % Spectr. C 13 % Spectr. |

-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
14630-40-1
Bis(trimethylsilyl)ethyne

-
A
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
B
-
74-86-2
acetylene

| Conditions | Yield |
|---|---|
| With HW(CO)3(C5H5) In dichloromethane-d2 at 22℃; for 0.5h; Product distribution; | A 100 % Spectr. B 48 % Spectr. |

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
115243-86-2, 115269-43-7, 115269-44-8, 115269-45-9, 120056-50-0, 120056-51-1
Acetic acid (2R,3R,4R,5R,6S)-3-acetoxy-2-acetoxymethyl-6-[(2S,4aS,8aR)-5-(2-furan-3-yl-ethyl)-1,1,4a,6-tetramethyl-1,2,3,4,4a,7,8,8a-octahydro-naphthalen-2-yloxy]-5-hydroxy-tetrahydro-pyran-4-yl ester

-
-
115243-90-8, 141978-30-5, 141978-31-6, 141978-32-7
baiyunyl 2-O-trimethylsilyl-3,4,6-tri-O-acetyl-α-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In dichloromethane for 10h; Ambient temperature; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
96429-43-5
(E)-6-((tert-butyldimethylsilyl)oxy)hex-3-en-2-one

-
-
116072-20-9
(E)-6-(tert-Butyl-dimethyl-silanyloxy)-2-trimethylsilanyloxy-hexa-1,3-diene

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether for 0.666667h; Ambient temperature; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
3152-46-3, 80779-43-7, 116405-08-4
(22E,24R)-3α,5-Cyclo-5α-ergost-7,22-dien-6-one

-
-
124696-42-0
(22E,24R)-6-Trimethylsiloxy-3α,5-cyclo-5α-ergost-6,22-diene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 0.333333h; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
64657-18-7
1,9-dideoxyforskolin

-
-
111455-15-3
7β-acetoxy-8,13-epoxy-6β-trimethylsilanyloxy-labd-14-en-11-one

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole In dichloromethane at 20℃; for 72h; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
141407-33-2
1-(phenylsulfinyl)hexan-2-one

-
-
141407-36-5
Trimethyl-{(E)-1-[1-phenylsulfanyl-meth-(E)-ylidene]-pent-2-enyloxy}-silane

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 2h; Mechanism; Ambient temperature; other β-keto sulfoxides; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 2h; Ambient temperature; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
141407-34-3
1-Benzenesulfinyl-decan-2-one

-
-
141420-65-7
Trimethyl-{(E)-1-[1-phenylsulfanyl-meth-(E)-ylidene]-non-2-enyloxy}-silane

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 2h; Ambient temperature; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
141407-35-4
1-Benzenesulfinyl-4-(4-methoxy-phenyl)-butan-2-one

-
-
141407-37-6
{(E)-3-(4-Methoxy-phenyl)-1-[1-phenylsulfanyl-meth-(E)-ylidene]-allyloxy}-trimethyl-silane

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 2h; Ambient temperature; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
102650-39-5
(3Z)-4-(benzoyloxy)-1-(α-furyl)-1-methoxy-3-<(trimethylsilyl)oxy>-1,3-butadiene

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether 1.) -78 deg C, 10 min; 2.) room temp., 0.5 h; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
153093-79-9
2,2-dimethyl-6-cycloundecene-4,8-diyn-1-one

-
-
153093-81-3
<(2,2-dimethyl-6,10-cycloundecdiene-4,8-diyn-1-yl)oxy>trimethylsilane

| Conditions | Yield |
|---|---|
| With TEA In dichloromethane for 5h; Ambient temperature; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
90-94-8
bis(p-dimethylaminophenyl)methanone

-
-
80239-31-2
methylen>(trimethylsilyl)oxonium-trifluormethansulfonat

| Conditions | Yield |
|---|---|
| In dichloromethane at 15℃; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
160072-66-2
(E)-2-(4-oxopent-2-en-1-yl)isoindoline-1,3-dione

-
-
160072-67-3
2-((E)-4-Trimethylsilanyloxy-penta-2,4-dienyl)-isoindole-1,3-dione

| Conditions | Yield |
|---|---|
| With triethylamine In benzene for 15h; Ambient temperature; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 25℃; for 1h; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
589-92-4
1-methylcyclohexan-4-one

-
-
38671-78-2
4-methyl-1-(trimethylsilyloxy)-1-cyclohexene

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; for 1.5h; | 100% |
| With triethylamine In dichloromethane for 1h; |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
185810-98-4
(1S,2S,3R,4aS,13bR,14aS)-3-(tert-Butyl-dimethyl-silanyloxy)-1-hydroxy-2,11-dimethoxy-5-oxo-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydro-indolo[2',3':3,4]pyrido[1,2-b]isoquinoline-1-carboxylic acid methyl ester

-
-
185811-02-3
(1S,2S,3R,4aS,13bR,14aS)-3-(tert-Butyl-dimethyl-silanyloxy)-2,11-dimethoxy-5-oxo-1-trimethylsilanyloxy-1,2,3,4,4a,5,7,8,13,13b,14,14a-dodecahydro-indolo[2',3':3,4]pyrido[1,2-b]isoquinoline-1-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In dichloromethane for 4h; Ambient temperature; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
225640-45-9
methyl (phenyl 2,3-di-O-benzyl-1-thio-β-D-galactopyranosid)uronate

-
-
225640-47-1
methyl (phenyl 2,3-di-O-benzyl-1-thio-4-O-trimethylsilyl-β-D-galactopyranosid)uronate

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In dichloromethane at -10℃; for 0.0833333h; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
225640-46-0
benzyl (phenyl 2,3-di-O-benzyl-1-thio-β-D-galactopyranosid)uronate

-
-
225640-48-2
benzyl (phenyl 2,3-di-O-benzyl-1-thio-4-O-trimethylsilyl-β-D-galactopyranosid)uronate

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In dichloromethane at -10℃; for 0.166667h; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
56354-75-7, 121773-36-2
Ethyl 2-methyl-3,3,3-trifluoropropionate

| Conditions | Yield |
|---|---|
| With triethylamine at 100℃; for 5h; Substitution; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
97042-79-0
1-ethoxycarbonyl-2-trimethylsilylaziridine

| Conditions | Yield |
|---|---|
| Ring cleavage; Substitution; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
265093-28-5
2-Methoxycarbonyl-2-dimethylphenylsilyl-1-phenylaziridine

| Conditions | Yield |
|---|---|
| Salt formation; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate


| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In tetrahydrofuran at -78 - 0℃; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
204862-91-9, 152646-80-5, 183244-55-5
2-(diphenylphosphinyl)-2'-(diphenylphosphanyl)-1,1'-binaphthalene

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
100-66-3
methoxybenzene

-
-
350693-36-6
1,1-diphenyl-3-(trimethylsilyl)-1-(trimethylsilyloxy)prop-2-yne

| Conditions | Yield |
|---|---|
| In dichloromethane at -78℃; for 3h; Friedel-Crafts reaction; | 100% |

-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
434321-76-3
1-fluoro-1-isopropyl-1-phenyl-3,3-bis-trifluoromethyl-2-oxa-1λ5-phospha-indan

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; | 100% |
| In diethyl ether at 20℃; for 0.333333h; |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
434321-65-0
1-ethylsulfanyl-1-fluoro-3,3-bis-trifluoromethyl-1-(2,4,6-triisopropyl-phenyl)-2-oxa-1λ5-phospha-indan

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
447408-34-6
8a-allyl-4-(tert-butyl-diphenyl-silanyloxy)-2-(toluene-4-sulfonyl)-3,4,4a,8a-tetrahydro-2H,5H-isoquinoline-1,6-dione

-
-
447408-36-8
8a-allyl-4-(tert-butyl-diphenyl-silanyloxy)-2-(toluene-4-sulfonyl)-6-trimethylsilanyloxy-3,4,4a,8a-tetrahydro-2H-isoquinolin-1-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0℃; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With tetrachlorosilane In dichloromethane; chloroform | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
779349-32-5
1-(2,2-dimethoxyethyl)-4-phenylcyclohexanol

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In dichloromethane at 0℃; for 0.5h; | 100% |
| Stage #1: trimethylsilyl trifluoromethanesulfonate; 1-(2,2-dimethoxyethyl)-4-phenylcyclohexanol With 2,6-dimethylpyridine In dichloromethane at 0℃; for 0.5h; Stage #2: With water In dichloromethane | 100% |

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In dichloromethane at -78℃; for 1h; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
812681-73-5
(3S,4S)-4-Acetyl-3-methoxy-1-(4-methoxy-phenyl)-azetidin-2-one

-
-
804563-07-3
(3S,4S)-3-Methoxy-1-(4-methoxy-phenyl)-4-(1-trimethylsilanyloxy-vinyl)-azetidin-2-one

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -78 - 20℃; for 2h; | 100% |
-
-
27607-77-8
trimethylsilyl trifluoromethanesulfonate

-
-
767319-38-0
(-)-(1Z,2S,3R,4S)-1-ethylidene-2,4-dimethyl-5-oxo-3-((1S)-1-phenylethoxy)-heptyl isobutyrate

-
-
847758-56-9
Isobutyric acid (Z)-(2S,3R,4S)-1-eth-(Z)-ylidene-2,4-dimethyl-3-((S)-1-phenyl-ethoxy)-5-trimethylsilanyloxy-hept-5-enyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 100% |
| With triethylamine In dichloromethane at -20 - 20℃; for 2h; |
Trimethylsilyl Trifluoromethanesulfonate Specification
The IUPAC name of this chemical is trimethylsilyl trifluoromethanesulfonate. With the CAS registry number 27607-77-8, it is also named as Methanesulfonic acid, 1,1,1-trifluoro-, trimethylsilyl ester. The product's categories are Biochemistry; Nucleosides, Nucleotides & Related Reagents; Protecting Agents for Hydroxyl and Amino Groups; Protecting Agents, Phosphorylating Agents & Condensing Agents; Protection & Derivatization Reagents (for Synthesis); Si (Classes of Silicon Compounds); Silicon Compounds (for Synthesis); Silyl Esters; Si-O Compounds; Synthetic Organic Chemistry. It is clear colourless to light brown fuming liquid which reacts violently with water. Additionally, this chemical should be stored at the temperature of 2-8 °C.
The other characteristics of Trimethylsilyl Trifluoromethanesulfonate can be summarized as:
(1)ACD/LogP: 2.54; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.54; (4)ACD/LogD (pH 7.4): 2.54; (5)#H bond acceptors: 3; (6)#H bond donors: 0; (7)#Freely Rotating Bonds: 2; (8)Index of Refraction: 1.379; (9)Molar Refractivity: 40.23 cm3; (10)Molar Volume: 174 cm3; (11)Polarizability: 15.95×10-24 cm3; (12)Surface Tension: 21.9 dyne/cm; (13)Enthalpy of Vaporization: 36.17 kJ/mol; (14)Vapour Pressure: 7.81 mmHg at 25°C; (15)Rotatable Bond Count: 2; (16)Exact Mass: 221.999376; (17)MonoIsotopic Mass: 221.999376; (18)Topological Polar Surface Area: 51.8; (19)Heavy Atom Count: 12; (20)Complexity: 244.
Preparation of Trimethylsilyl Trifluoromethanesulfonate:
It can be obtained by hexamethyl-disiloxane and trifluoro-methanesulfonic acid anhydride. This reaction must react at temperature of 70-75°C for 2 hours. The yield is 88%.
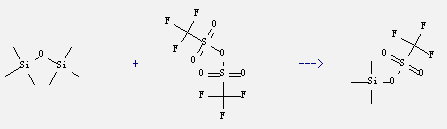
Uses of Trimethylsilyl Trifluoromethanesulfonate:
It is cationic initiator which is used as catalysts and silicon reagent in organic synthesis. It also can react with 1,2-epoxy-cyclopentane to get (2-cyclopenten-1-yloxy)trimethylsilane. This reaction needs reagent DBU and solvent benzene.
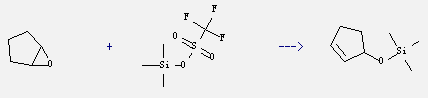
Safety information of Trimethylsilyl Trifluoromethanesulfonate:
When you are using this chemical, please be cautious about it as the following:It is flammable, so people should keep it away from sources of ignition. And it can cause burns. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you want to contact this product, you must wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
People can use the following data to convert to the molecule structure.
1. SMILES:C[Si](C)(C)OS(=O)(=O)C(F)(F)F
2. InChI:InChI=1/C4H9F3O3SSi/c1-12(2,3)10-11(8,9)4(5,6)7/h1-3H3
3. InChIKey:FTVLMFQEYACZNP-UHFFFAOYAR
Related Products
- Trimethylsilyl 2-(fluorosulfonyl)difluoroacetate
- Trimethylsilyl acetate
- Trimethylsilyl bromoacetate
- Trimethylsilyl chlorosulphate
- Trimethylsilyl cyanide
- Trimethylsilyl isobutyrate
- Trimethylsilyl methacrylate
- Trimethylsilyl methanesulfonate
- Trimethylsilyl perchlorate
- Trimethylsilyl propionate
- 27607-78-9
- 27608-03-3
- 2760-98-7
- 27610-45-3
- 27610-62-4
- 27610-92-0
- 27612-67-5
- 27613-78-1
- 27618-25-3
- 27620-10-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View