-
Name
Methylurea
- EINECS 209-935-0
- CAS No. 598-50-5
- Article Data93
- CAS DataBase
- Density 1.041 g/cm3
- Solubility 1000 g/L (20 ºC)
- Melting Point ~93 °C
- Formula C2H6N2O
- Boiling Point 114.6 °C at 760 mmHg
- Molecular Weight 74.0824
- Flash Point 23.1 °C
- Transport Information
- Appearance White, crystalline needles.
- Safety 22-45-36/37
- Risk Codes 68-37-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Urea,methyl- (6CI,8CI,9CI);1-Methylurea;Methylurea;Monomethylurea;N-Methylurea;
- PSA 55.12000
- LogP 0.37570
Synthetic route

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In water at 0 - 20℃; for 3.5h; | 99% |
| With diethyl ether; ammonia | |
| With ammonia at 45℃; |

| Conditions | Yield |
|---|---|
| With ammonia In water at 65℃; | A n/a B 96% |
-
-
22013-97-4
N-methyl-thiocarbamic acid S-methyl ester

-
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In 1,4-dioxane | 96% |
| With ammonium hydroxide In 1,4-dioxane at 65℃; for 3h; |

| Conditions | Yield |
|---|---|
| In water for 0.5h; Heating; | 90% |
-
-
1142408-78-3
1,5-dimethyl-3-(3,4,5-trimethoxyphenyl)-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one

-
A
-
1142408-82-9
1-methyl-5,6-dioxo-3-(3,4,5-trimethoxyphenyl)-1,4,5,6-tetrahydro-1,2,4-triazine

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dimethyl-3-(3,4,5-trimethoxyphenyl)-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 89% B n/a |

| Conditions | Yield |
|---|---|
| With tetrabutylammonium periodite In dichloromethane; acetonitrile at 20℃; for 0.583333h; | 88% |
| With sodium bromate at 25℃; Rate constant; Mechanism; various stoichiometry; also in the presence of acid; |
-
-
1142408-77-2
1,5-dimethyl-3-p-tolyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one

-
A
-
1142408-80-7
1-methyl-5,6-dioxo-3-p-tolyl-1,4,5,6-tetrahydro-1,2,4-triazine

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dimethyl-3-p-tolyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 87% B n/a |

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 3-(4-bromophenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 86% B n/a |
-
-
61602-19-5
1,5-dimethyl-3-phenyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dimethyl-3-phenyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 84% B 16 mg |
-
-
1142408-75-0
3-(4-chlorophenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 3-(4-chlorophenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 83% B 20 mg |
-
-
61602-21-9
3-(4-methoxyphenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one

-
A
-
1142408-81-8
3-(4-methoxyphenyl)-1-methyl-5,6-dioxo-1,4,5,6-tetrahydro-1,2,4-triazine

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 3-(4-methoxyphenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 83% B n/a |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 5h; Inert atmosphere; | 78% |
| With N,N,N',N'-tetramethylguanidinium acetate In water at 60℃; for 0.5h; |

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 3-(3,4-dimethoxyphenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 78% B n/a |
-
-
1142408-76-1
3-(4-hydroxyphenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 3-(4-hydroxyphenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 76% B n/a |
-
-
1142408-74-9
3-(4-fluorophenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 3-(4-fluorophenyl)-1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 72% B n/a |
-
-
123-75-1
pyrrolidine

-
-
17758-39-3
1-methyl-5-nitro-1H-pyrimidin-2-one

-
A
-
3760-54-1
1-pyrrolidinecarboxaldehyde

-
C
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 168h; | A 41 %Spectr. B 68% C 29 %Spectr. |

-
-
100422-34-2
theobromuric acid

-
A
-
3659-97-0
1-methylparabanic acid

-
B
-
100422-43-3
ω.ω'-Dimethyl-carbonyldiharnstoff

-
C
-
144-62-7
oxalic acid

-
D
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| With hydrogenchloride | A n/a B 50% C n/a D n/a |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; Carbonate buffer In ethanol at 35℃; Irradiation; | A 41.5% B n/a |
-
-
1142408-71-6
1,3,5-trimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-trimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 41% B n/a |
-
-
106284-73-5
1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dimethyl-1H-imidazo[4,5-e][1,2,4]triazin-6(5H)-one With ethanol; sodium hydroxide Reflux; Stage #2: With hydrogenchloride In ethanol; water pH=7; Cooling with ice; | A 36% B n/a |
-
-
60782-14-1, 60782-15-2
(4S,6S)-5,5-Diallyl-4,6-dihydroxy-1-methyl-tetrahydro-pyrimidin-2-one

-
B
-
78959-59-8
2,2-Diallyl-3-(3-methylureido)-1-propanol

-
C
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In 1,4-dioxane; water for 4h; | A n/a B 25% C n/a |


| Conditions | Yield |
|---|---|
| With alkali | |
| With barytes | |
| With water |
-
-
861346-33-0
1-methyl-2,5-dioxo-imidazolidine-4-carboxylic acid methylamide

-
A
-
144-62-7
oxalic acid

-
B
-
74-89-5
methylamine

-
C
-
598-50-5
N-Methylurea


| Conditions | Yield |
|---|---|
| With nitric acid Erhitzen zum Sieden; | |
| With potassium hydroxide at 25℃; Rate constant; |
-
-
26891-98-5
(Methylcarbamoyl)azid

-
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| With ammonia |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
A
-
2757-85-9
1,3-dimethylalloxan

-
B
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| With chloramine-B In hydrogenchloride at 29.9℃; Kinetics; Mechanism; Thermodynamic data; it was investigated the effect of concentration of reactants, ionic strength, temperature.; | |
| With hydrogenchloride; potassium chlorate at 50℃; | |
| With hydrogenchloride; N-bromo-p-toluenesulfonamide at 14.85℃; Kinetics; Further Variations:; pH-values; Reagents; Solvents; Temperatures; Oxidation; | |
| With hydrogenchloride; sodium perchlorate; bromamine B at 14.85℃; Kinetics; Oxidation; |

| Conditions | Yield |
|---|---|
| With carbon dioxide; water; lead dioxide |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 5h; | 100% |
| With hydrogenchloride; ethanol | |
| With toluene-4-sulfonic acid In toluene for 3h; Condensation; Heating; | |
| In acetonitrile at 20℃; for 1.5h; |
-
-
610-94-6
2-bromobenzoic acid methyl ester

-
-
598-50-5
N-Methylurea

-
-
607-19-2
3-methyl-2,4(1H,3H)-quinazolinedione

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; for 48h; | 99% |
| With tris-(dibenzylideneacetone)dipalladium(0); caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1,4-dioxane at 100℃; for 24h; | 61% |

| Conditions | Yield |
|---|---|
| Stage #1: N-Methylurea With sodium ethanolate In ethanol at 20℃; Reflux; Stage #2: ethyl 2-cyanoacetate In ethanol for 5h; Reagent/catalyst; Solvent; | 98.2% |
| With sodium ethanolate In ethanol for 6h; Heating; | 95% |
| Stage #1: ethyl 2-cyanoacetate With sodium ethanolate at 20℃; for 0.333333h; Stage #2: N-Methylurea for 3h; Reflux; Stage #3: With sulfuric acid In water for 2h; pH=7; | 95% |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 66℃; for 24h; Solvent; Reagent/catalyst; Temperature; | 98.1% |
| With sodium hydroxide In propan-1-ol for 8h; Reflux; | 91.2% |
-
-
141-97-9
ethyl acetoacetate

-
-
100-52-7
benzaldehyde

-
-
598-50-5
N-Methylurea

-
-
123044-27-9, 50628-42-7
ethyl 1,6-dimethyl-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With brominated Amberlyst 15 In acetonitrile for 1h; Biginelli Pyrimidone Synthesis; Reflux; | 98% |
| With polyphosphate ester In tetrahydrofuran for 15h; Heating; | 95% |
| With myo-inositol 1,2,3,4,5,6-hexakisphosphate In neat (no solvent) at 100℃; for 0.6h; Biginelli Pyrimidone Synthesis; Green chemistry; | 94% |


| Conditions | Yield |
|---|---|
| With chitosan decorated Fe3O4 magnetic nanoparticles In ethanol; water at 60℃; for 0.125h; Green chemistry; | 98% |
| at 145℃; | |
| With potassium hydroxide | |
| at 145℃; |
-
-
302-17-0
chloral hydrate

-
-
598-50-5
N-Methylurea

-
-
1954-79-6
N-methyl-N'-(2,2,2-trichloro-1-hydroxyethyl)urea

| Conditions | Yield |
|---|---|
| In water for 48h; Substitution; | 97% |
| With water |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 22℃; for 2h; | 97% |
-
-
102-01-2
N-phenylacetoacetamide

-
-
591-31-1
3-methoxy-benzaldehyde

-
-
598-50-5
N-Methylurea

-
-
1255416-25-1
4-(3-methoxyphenyl)-1,6-dimethyl-5-(N-phenylcarboxamido)-2-oxo-1,2,3,4-tetrahydropyrimidine

| Conditions | Yield |
|---|---|
| With chloroacetic acid at 90℃; Biginelli reaction; Neat (no solvent); | 97% |
| at 120 - 150℃; | 62% |

-
-
1226-42-2
1,2-bis(4-methoxyphenyl)-1,2-ethanedione

-
-
598-50-5
N-Methylurea

-
-
66242-67-9
5,5-bis-(4-methoxy-phenyl)-3-methyl-imidazolidine-2,4-dione

| Conditions | Yield |
|---|---|
| With chitosan decorated Fe3O4 magnetic nanoparticles In ethanol; water at 60℃; for 0.133333h; Green chemistry; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: N-Methylurea With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 3h; Inert atmosphere; Stage #2: diethyl dibutylmalonate In N,N-dimethyl-formamide; mineral oil at 0 - 60℃; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With silica gel-supported polyphosphoric acid at 120℃; for 0.0833333h; | 96% |
| With Indion-130 resin at 110℃; for 0.1h; | 94% |
| With sulfanilic acid functionalized silica coated Fe3O4 nanoparticles In neat (no solvent) at 120℃; for 0.166667h; Green chemistry; | 94% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride at 100℃; for 1h; Biginelli condensation; | 96% |
| With hydrogenchloride at 100℃; for 1h; Biginelli condensation; | 96% |

-
-
100-52-7
benzaldehyde

-
-
105-45-3
acetoacetic acid methyl ester

-
-
598-50-5
N-Methylurea

-
-
302932-49-6
methyl 1,6-dimethyl-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| With myo-inositol 1,2,3,4,5,6-hexakisphosphate In neat (no solvent) at 100℃; for 0.5h; Biginelli Pyrimidone Synthesis; Green chemistry; | 96% |
| With toluene-4-sulfonic acid In methanol for 16h; Reflux; Inert atmosphere; | 90% |
| With cerous p-toluenesulfonate tetrahydrate at 80℃; for 0.0833333h; Green chemistry; | 88% |
| With yttrium (III) acetate hydrate; acetic acid at 115℃; for 4.5h; Biginelli reaction; | 86% |
| With toluene-4-sulfonic acid In ethanol Reflux; Inert atmosphere; |

-
-
105-45-3
acetoacetic acid methyl ester

-
-
6630-33-7
ortho-bromobenzaldehyde

-
-
598-50-5
N-Methylurea

-
-
923235-54-5
5-methoxycarbonyl-1,6-dimethyl-4-(2-bromophenyl)-3,4-dihydropyrimidin-2(1H)-one

| Conditions | Yield |
|---|---|
| With yttrium (III) acetate hydrate; acetic acid at 115℃; for 4.5h; Biginelli reaction; | 96% |

-
-
108-24-7
acetic anhydride

-
-
118-92-3
anthranilic acid

-
-
598-50-5
N-Methylurea

-
-
1377584-86-5
N,2-dimethyl-4-oxoquinazoline-3(4H)-carboxamide

| Conditions | Yield |
|---|---|
| With titanium dioxide nanoparticles at 80℃; for 10h; Neat (no solvent); | 96% |

-
-
55114-30-2
2-benzyl-2-methylmalonic acid diethyl ester

-
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| Stage #1: N-Methylurea With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.166667h; Inert atmosphere; Stage #2: 2-benzyl-2-methylmalonic acid diethyl ester In N,N-dimethyl-formamide at 0 - 20℃; for 18h; | 96% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In acetonitrile at 70℃; for 8h; Temperature; Solvent; | 96% |
-
-
1076-38-6
4-hydroxy[1]benzopyran-2-one

-
-
122-51-0
orthoformic acid triethyl ester

-
-
598-50-5
N-Methylurea

-
-
134297-75-9
3-methylureidomethylene-4-oxo-coumarin

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 2h; Heating; | 95% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol for 6h; Heating; | 95% |


| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid at 125℃; for 5h; | 95% |
| With Indion-130 resin at 110℃; for 0.166667h; | 91% |
| With silica gel-supported polyphosphoric acid at 120℃; for 0.133333h; | 86% |
| With barium phosphate In neat (no solvent) at 100℃; for 0.25h; | 77% |


| Conditions | Yield |
|---|---|
| With silica gel-supported polyphosphoric acid at 120℃; for 0.0666667h; | 95% |
| With sulfanilic acid functionalized silica coated Fe3O4 nanoparticles In neat (no solvent) at 120℃; for 0.2h; Green chemistry; | 92% |
| With Indion-130 resin at 110℃; for 0.2h; | 90% |
| toluene-4-sulfonic acid at 125℃; for 6h; | 89% |
| With barium phosphate In neat (no solvent) at 100℃; for 0.333333h; | 82% |

-
-
731857-74-2
C15H5F17O4S

-
-
105-45-3
acetoacetic acid methyl ester

-
-
598-50-5
N-Methylurea

-
-
1350738-43-0
C22H15F17N2O6S

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate In acetonitrile at 120℃; for 0.333333h; Biginelli reaction; Microwave irradiation; | 95% |

-
-
141-97-9
ethyl acetoacetate

-
-
873-76-7
para-Chlorobenzyl alcohol

-
-
598-50-5
N-Methylurea

-
-
302821-62-1
ethyl 4-(4-chlorophenyl)-1,6-dimethyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: para-Chlorobenzyl alcohol With Oxone; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; potassium bromide In neat (no solvent) for 0.5h; Biginelli Pyrimidone Synthesis; Milling; Green chemistry; Stage #2: ethyl acetoacetate; N-Methylurea In neat (no solvent) for 3.5h; Biginelli Pyrimidone Synthesis; Milling; Green chemistry; regioselective reaction; | 95% |


-
-
598-50-5
N-Methylurea

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 20h; Reflux; regioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With cerous p-toluenesulfonate tetrahydrate at 80℃; for 0.166667h; Green chemistry; | 95% |


| Conditions | Yield |
|---|---|
| With cerium(III) p-toluenesulfonate tetrahydrate In neat (no solvent) at 80℃; for 1.7h; Biginelli Pyrimidone Synthesis; Green chemistry; | 95% |

Methylurea Consensus Reports
Methylurea Specification
The CAS registry number of N-Methylurea is 598-50-5. The IUPAC name is methylurea. Its EINECS registry number is 209-935-0 . In addition, the molecular formula is C2H6N2O and the molecular weight is 74.08. It is a kind of white to off-white crystalline solid and belongs to the classes of Carbonyl Compounds; Organic Building Blocks; Ureas.
Physical properties about this chemical are: (1)ACD/LogP: -1.23; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.23; (4)ACD/LogD (pH 7.4): -1.23; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 5.12; (8)ACD/KOC (pH 7.4): 5.12; (9)#H bond acceptors: 3; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 23.55 Å2; (13)Index of Refraction: 1.432; (14)Molar Refractivity: 18.47 cm3; (15)Molar Volume: 71.1 cm3; (16)Polarizability: 7.32 ×10-24cm3; (17)Surface Tension: 35.5 dyne/cm; (18)Density: 1.041 g/cm3; (19)Flash Point: 23.1 °C; (20)Enthalpy of Vaporization: 35.3 kJ/mol; (21)Boiling Point: 114.6 °C at 760 mmHg ; (22)Vapour Pressure: 19.8 mmHg at 25°C.
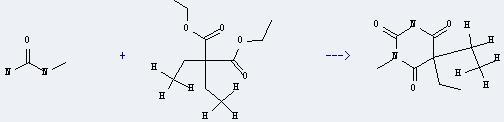
Uses of N-Methylurea: it can be used in organic synthesis and pharmaceutical industry. And it can react with diethylmalonic acid diethyl ester to get 5,5-diethyl-1-methyl-pyrimidine-2,4,6-trione. This reaction will need reagents Na and EtOH. The reaction time is 4 hours at reaction temperature of 115 °C. The yield is about 68%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. And it is irritating to the respiratory system. It has risk of irreversible effects possibly. During using it, wear suitable protective clothing and gloves and do not breathe dust. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.).
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(N)NC
(2)InChI: InChI=1/C2H6N2O/c1-4-2(3)5/h1H3,(H3,3,4,5)
(3)InChIKey: XGEGHDBEHXKFPX-UHFFFAOYAA
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | parenteral | 10189mg/kg (10189mg/kg) | BEHAVIORAL: GENERAL ANESTHETIC BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Journal of Pharmacology and Experimental Therapeutics. Vol. 52, Pg. 216, 1934. |
| rat | LD50 | intraperitoneal | > 2gm/kg (2000mg/kg) | Naunyn-Schmiedebergs Archiv fuer Pharmakologie und Experimentelle Pathologie. Vol. 257, Pg. 296, 1967. | |
| rat | LDLo | oral | 500mg/kg (500mg/kg) | National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review. Vol. 5, Pg. 47, 1953. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View