-
Name
Allyl bromide
- EINECS 203-446-6
- CAS No. 106-95-6
- Article Data129
- CAS DataBase
- Density 1.398 g/cm3
- Solubility insoluble in water
- Melting Point -119 ºC
- Formula C3H5Br
- Boiling Point 68.107 ºC at 760 mmHg
- Molecular Weight 120.977
- Flash Point -1 ºC
- Transport Information UN 1099 3/PG 1
- Appearance Colorless liquid
- Safety 16-26-36/37/39-45-60-61
- Risk Codes 11-23/25-34-50
-
Molecular Structure
-
Hazard Symbols
 F,
F, T,
T, N
N
- Synonyms Propene,3-bromo- (8CI);1-Bromo-2-propene;2-Propenyl bromide;3-Bromo-1-propene;3-Bromopropene;3-Bromopropylene;NSC 7596;
- PSA 0.00000
- LogP 1.56730
Synthetic route

| Conditions | Yield |
|---|---|
| With CO In decane (CO); heating (6 h, 140°C), cooling; chromy. (silica gel, ether); | A 97% B n/a |
| With CO In decane (CO); heating (2 h, 140°C), cooling; chromy. (silica gel, ether); | A 48% B n/a |

| Conditions | Yield |
|---|---|
| With CO In decane (CO); heating (2 h, 140°C), cooling; chromy. (silica gel, ether); | A 96% B n/a |
| With CO In decane (CO); heating (2 h, 120°C), cooling; chromy. (silica gel, ether); | A 45% B n/a |
| With carbon monoxide In diethylene glycol heating in diglime under CO atm.;; | A 70-80 B 50-70 |

| Conditions | Yield |
|---|---|
| (N2); heating (16 h, 140°C), cooling; concn., chromy. (silica gel, MeOH); elem. anal.; | A 94% B n/a |


| Conditions | Yield |
|---|---|
| With para-bromotoluene; oxalic acid at 65℃; for 1.33333h; Temperature; Reflux; | 93% |

| Conditions | Yield |
|---|---|
| With piperazine; hydrogen In ethanol at 80℃; under 4500.45 Torr; for 24h; | 92% |
| With piperazine; hydrogen In ethanol at 100℃; under 4500.45 Torr; for 24h; Green chemistry; | |
| With hydrogen In ethanol at 100℃; under 4500.45 Torr; for 24h; chemoselective reaction; | 89 %Chromat. |
| With hydrogen In ethanol at 30℃; under 3750.38 Torr; for 2h; chemoselective reaction; |

| Conditions | Yield |
|---|---|
| With silica bromide In dichloromethane at 20℃; for 0.0833333h; | 91% |
| With Silphos; bromine In acetonitrile for 0.166667h; Heating; | 84% |
| With tetradecafluorohexane; phosphorus tribromide In diethyl ether at 20℃; for 12h; | 75% |

| Conditions | Yield |
|---|---|
| With hexaethylphosphoric triamide In benzene for 24h; Ambient temperature; Yields of byproduct given; | A 89.9% B n/a |
-
-
369594-22-9
6,7-dihydropyrido[3,2,1-ij]quinazoline-1,3 (2H,5H)-dione

-
A
-
1422210-23-8
2-allyl-6,7-dihydro-5H-pyrido[3,2,1-ij]quinazoline-1,3-dione

-
B
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| A n/a B 89% |
-
-
76065-47-9
C17H19BrO2Te

-
A
-
24727-22-8
dibromo-bis-(4-methoxy-phenyl)-λ4-tellane

-
B
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| With bromine at 0℃; for 4h; | A 80% B n/a |
-
-
107-05-1
3-chloroprop-1-ene

-
A
-
78-75-1
1,2-Dibromopropane

-
B
-
3017-96-7
1-bromo-2-chloropropane

-
C
-
3017-95-6, 127054-44-8, 130232-86-9
2-bromo-1-chloropropane

-
D
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; ferric(III) bromide In dichloromethane at 25℃; for 0.5h; | A 79% B 16% C 2% D 2% |

-
-
498-62-4
3-thiophene carboxaldehyde

-
-
100-39-0
benzyl bromide

-
-
123-11-5
4-methoxy-benzaldehyde

-
A
-
106-95-6
allyl bromide

-
B
-
220338-01-2
3-((Z)-2-Benzylsulfanyl-vinyl)-5-(4-methoxy-phenyl)-2,5-dihydro-furan-2-ol

| Conditions | Yield |
|---|---|
| With samarium; 1,2-Diiodoethane In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide for 27h; Ambient temperature; | A 22% B 63% |

-
-
506-68-3
bromocyane

-
-
76065-43-5
allyldiphenyltelluronium bromide

-
A
-
76065-55-9
C13H10BrNTe

-
B
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| at 25℃; for 8h; | A 62% B n/a |

-
-
18146-00-4
allyloxytrimethylsilane

-
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| With Tri-n-butylfluorphosphoniumbromid In dichloromethane at 20℃; for 24h; | 61% |
-
-
105900-07-0
trans-{Me2Co(11-hydroxy-2,3,9,10-tetramethyl-1,4,8,11-tetraazaundeca-1,3,8,10-tetraen-1-olate)}

-
-
100-39-0
benzyl bromide

-
A
-
103-29-7
1,1'-(1,2-ethanediyl)bisbenzene

-
B
-
106-95-6
allyl bromide

-
C
-
108-88-3
toluene

| Conditions | Yield |
|---|---|
| In [D3]acetonitrile Kinetics; Irradiation (UV/VIS); Degasses condition, 298 K, 33 h;; detected by NMR-spectroscopy;; | A 22% B n/a C 55% |

-
-
25360-57-0
{EtCobis(dimethylglyoximato)(pyridine)}

-
-
100-39-0
benzyl bromide

-
A
-
106-95-6
allyl bromide

-
B
-
108-88-3
toluene

| Conditions | Yield |
|---|---|
| In [D3]acetonitrile Kinetics; Irradiation (UV/VIS); Degasses condition, 298 K, 14 h;; detected by NMR-spectroscopy;; | A n/a B 33% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; cetylpyridinium bromide at 130℃; for 2h; | 20% |
| With silver(l) oxide | |
| With Hexamethylphosphorous triamide |
-
-
23642-14-0
{MeCobis(dimethylglyoximato)(pyridine)}

-
-
100-39-0
benzyl bromide

-
A
-
106-95-6
allyl bromide

-
B
-
108-88-3
toluene

| Conditions | Yield |
|---|---|
| In [D3]acetonitrile Kinetics; Irradiation (UV/VIS); Degasses condition, 298 K, 71 h;; detected by NMR-spectroscopy;; | A n/a B 6.4% |

-
-
187737-37-7
propene

-
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| With bromine at 300 - 315℃; | |
| With N-Bromosuccinimide; toluene-4-sulfonic acid In tetrahydrofuran at 100℃; for 4h; Inert atmosphere; Schlenk technique; | |
| With bromine at 525℃; |
-
-
506-68-3
bromocyane

-
-
31930-96-8
N-allyl-N-benzylaniline

-
A
-
25855-25-8
N-benzyl-N-phenylcyanamide

-
B
-
106-95-6
allyl bromide


| Conditions | Yield |
|---|---|
| With hydrogen bromide; zinc Darst.; |

| Conditions | Yield |
|---|---|
| With triphenyl phosphite at 140℃; |
-
-
110-52-1
1,4-dibromo-butane

-
-
34557-54-5
methane

-
-
14604-48-9
vinyl cation

-
A
-
187737-37-7
propene

-
B
-
463-49-0
1,2-propanediene

-
C
-
106-94-5
propyl bromide

-
D
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| at 100℃; under 60 - 720 Torr; Product distribution; Kinetics; Thermodynamic data; labelled with tritium; -ΔH; |

-
-
110-52-1
1,4-dibromo-butane

-
-
463-49-0
1,2-propanediene

-
A
-
557-93-7
2-bromoprop-1-ene

-
B
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| With water; hydrogen; oxygen at 37℃; under 150 Torr; Product distribution; Irradiation; variation of system composition and initial pressure; |


| Conditions | Yield |
|---|---|
| Mechanism; Irradiation; |

-
-
1981-80-2, 13932-24-6
allyl radical

-
-
106-95-6
allyl bromide

| Conditions | Yield |
|---|---|
| With bromine under 1 Torr; Rate constant; concentration dependence; reaction of allyl radicals with NO2; | |
| With bromine at 24.9 - 258.9℃; Kinetics; Irradiation; carrier gas, He, the role of resonance stabilization; |

| Conditions | Yield |
|---|---|
| With pyridine; tributyltin bromide at 50℃; Thermodynamic data; Equilibrium constant; Δ G; | 20 % Chromat. |
| With bismuth(III) bromide In 1,2-dichloro-ethane for 1.75h; Heating; Yield given; |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Heating; | 100% |
| With sodium hydroxide; tetrabutylammomium bromide In dichloromethane; water at 25℃; for 4h; | 84% |
| With potassium carbonate In ethanol at 70℃; for 10h; regioselective reaction; | 84% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 5h; Heating; | 100% |
| Stage #1: 7-hydroxy-2H-chromen-2-one With potassium carbonate In acetone at 20℃; for 0.0833333h; Stage #2: allyl bromide In acetone for 3h; Reflux; | 100% |
| With potassium carbonate In acetone at 70℃; for 12h; | 98.6% |
-
-
90-33-5, 79566-13-5
7-hydroxy-4-methyl-chromen-2-one

-
-
106-95-6
allyl bromide

-
-
3993-57-5
7-allyloxy-4-methyl-2H-chromen-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 60℃; for 1h; regioselective reaction; | 100% |
| With potassium carbonate In acetone for 16h; Reflux; | 90% |
| With potassium carbonate In acetone for 12h; Reflux; | 90% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In various solvent(s) for 2.5h; Ambient temperature; | 100% |
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In dichloromethane for 3h; Product distribution; Ambient temperature; other solvent; | 90% |
| 87% | |
| With sodium hydroxide; tetrabutylammomium bromide In toluene | 62% |
| With copper; sodium carbonate; benzene |

| Conditions | Yield |
|---|---|
| With ammonium acetate; zinc In tetrahydrofuran at 0℃; for 0.166667h; Inert atmosphere; | 100% |
| zinc In N,N-dimethyl-formamide for 2h; Product distribution; Ambient temperature; other solvents : N,N-dimethylacetoamide, N-methyl-2-pyrrolidone, 2-pyrrolidone, 2-methyloxazoline; | 99% |
| With zinc In N,N-dimethyl-formamide for 2h; Ambient temperature; other solvents : N,N-dimethylacetoamide, N-methyl-2-pyrrolidone, 2-pyrrolidone, 2-methyloxazoline; | 99% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In water; N,N-dimethyl-formamide at 20℃; for 12h; | 100% |
| With potassium carbonate In acetone at 20℃; for 24h; | 99% |
| With potassium carbonate In acetone at 20℃; for 20h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With zinc In tetrahydrofuran at 20℃; for 1h; Alkylation; Barbier allylation; | 100% |
| With potassium fluoride; antimony In water for 16h; allylation; | 100% |
| With hydrogenchloride; antimony In water for 16h; Product distribution; Further Variations:; Reagents; times; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 65℃; | 100% |
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 6h; | 100% |
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 20℃; for 6h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 9h; Reflux; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1h; | 100% |
-
-
148-53-8
3-methoxy-2-hydroxybenzaldehyde

-
-
106-95-6
allyl bromide

-
-
23343-06-8
2-allyloxy-3-methoxy-benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 50℃; Williamson Ether Synthesis; | 100% |
| With sodium hydroxide; benzyltri(n-butyl)ammonium chloride In dichloromethane Ambient temperature; | 98% |
| With potassium carbonate In acetonitrile for 4h; Inert atmosphere; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 20℃; | 100% |
| With silica gel In water at 20℃; for 1h; | 95% |
| With potassium fluoride on Celite In acetonitrile at 83℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 18h; Heating; | 100% |
| With potassium carbonate; sodium iodide In ethanol for 3h; Reflux; | 100% |
| With potassium carbonate In ethanol for 3h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In butanone for 12h; Heating; | 100% |
| With potassium carbonate In acetone at 20℃; Reflux; | 100% |
| With potassium carbonate In acetone Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; Reflux; | 100% |
| With potassium carbonate; potassium iodide In acetone for 18h; Reflux; | 100% |
| With potassium carbonate | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 65℃; for 20h; | 100% |
| With potassium carbonate In dimethyl sulfoxide at 20℃; for 8h; | 99% |
| With potassium carbonate In acetone Heating; | 89% |

| Conditions | Yield |
|---|---|
| In ethanol at 95℃; for 48h; | 100% |
| for 2h; Reflux; | 92% |

| Conditions | Yield |
|---|---|
| With ammonium acetate; zinc In tetrahydrofuran at 0℃; for 0.0166667h; Barbier reaction; | 100% |
| With manganese; chloro-trimethyl-silane; indium In tetrahydrofuran at 20℃; for 4h; Alkylation; | 98% |
| With manganese; chloro-trimethyl-silane; indium In tetrahydrofuran at 20℃; for 4h; Barbier allylation; | 98% |

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In tetrahydrofuran for 1h; Ambient temperature; | 100% |
| With silica gel; ammonium chloride; zinc for 16h; Product distribution; Mechanism; Ambient temperature; other carbonyl compounds; other allyl halides; var. solid or liquid phases; var. reaction time; | 98% |
| With chloro-trimethyl-silane; Piperonyl butoxide; tetrabutylammomium bromide In N,N-dimethyl-formamide for 2h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; Alkylation; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 0.833333h; | 99% |

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-4-methyl-phenol at 115℃; for 16h; | 100% |
| for 16h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; Reflux; | 100% |
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In acetone for 1h; Reflux; Inert atmosphere; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 24h; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 2h; Inert atmosphere; | 99% |
-
-
93-51-6
2-Methoxy-4-methylphenol

-
-
106-95-6
allyl bromide

-
-
201359-55-9
1-(allyloxy)-2-methoxy-4-methylbenzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 12h; Heating; | 100% |
| With potassium carbonate; sodium iodide In acetone for 15h; Reflux; Inert atmosphere; | 96% |
| With potassium carbonate In acetone for 6h; Heating; | 95% |
-
-
529-35-1
5,6,7,8-Tetrahydronaphthalen-1-ol

-
-
106-95-6
allyl bromide

-
-
107203-35-0
5-(allyloxy)-1,2,3,4-tetrahydronaphthalene

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 25℃; for 2h; Inert atmosphere; | 100% |
| With methanol; sodium methylate | |
| With sodium; toluene at 70 - 80℃; | |
| With potassium carbonate; acetone |
-
-
3251-56-7
2-methoxy-4-nitrophenol

-
-
106-95-6
allyl bromide

-
-
99060-58-9
1-(allyloxy)-2-methoxy-4-nitrobenzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 55℃; for 12h; | 100% |
| With potassium carbonate; acetone | |
| at 70℃; for 6h; |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium hydroxide In 2-methyltetrahydrofuran; water for 0.5h; Reflux; | 100% |
| With potassium carbonate In acetone for 1h; Reflux; | 98% |
| Stage #1: allyl bromide With sodium hydride In N,N-dimethyl-formamide; oil at -10℃; for 0.166667h; Stage #2: p-Iodophenol In N,N-dimethyl-formamide; oil for 0.75h; | 96% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 6h; Heating / reflux; | 100% |
| With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In dichloromethane for 1.5h; Ambient temperature; | 100% |
| With sodium hydroxide In water for 2h; | 70% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In dichloromethane; water for 1h; Schlenk technique; Inert atmosphere; | 63% |
| With lithium methanolate |
-
-
13319-71-6
2-bromo-6-methylphenol

-
-
106-95-6
allyl bromide

-
-
854260-75-6
2-(allyloxy)-1-bromo-3-methylbenzene

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-6-methylphenol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 0.0833333h; Stage #2: allyl bromide In N,N-dimethyl-formamide; mineral oil at 20℃; | 100% |
| With potassium carbonate; acetone | |
| Stage #1: 2-bromo-6-methylphenol With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; Stage #2: allyl bromide In N,N-dimethyl-formamide; mineral oil at 0 - 25℃; Stage #3: With water In N,N-dimethyl-formamide; mineral oil |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 20℃; Reflux; | 100% |
| With potassium carbonate In acetone for 8h; Heating; | 95% |
| With potassium carbonate In ethanol for 3h; Reflux; Inert atmosphere; | 93% |
Allyl bromide Specification
The 3-Bromopropene, with the CAS registry number 106-95-6, is also known as Allyl bromide. It belongs to the product categories of Pharmaceutical Intermediates; Omega-Unsaturated Halides; Biochemistry; Omega-Functional Alkanols, Carboxylic Acids, Amines & Halides; Reagents for Oligosaccharide Synthesis. Its EINECS registry number is 203-446-6. This chemical's molecular formula is C3H5Br and molecular weight is 120.98. What's more, both its IUPAC name and systematic name are the same which is called 3-Bromoprop-1-ene. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, allyls and other organic compounds. Physically, 3-Bromopropene is a clear liquid with an intense, acrid, and persistent smell.
Physical properties about 3-Bromopropene are: (1)ACD/LogP: 1.79; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.79; (4)ACD/LogD (pH 7.4): 1.79; (5)ACD/BCF (pH 5.5): 13.502; (6)ACD/BCF (pH 7.4): 13.502; (7)ACD/KOC (pH 5.5): 224.264; (8)ACD/KOC (pH 7.4): 224.264; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 0 Å2; (13)Index of Refraction: 1.455; (14)Molar Refractivity: 23.405 cm3; (15)Molar Volume: 86.331 cm3; (16)Surface Tension: 24.797 dyne/cm; (17)Density: 1.401 g/cm3; (18)Enthalpy of Vaporization: 30.24 kJ/mol; (19)Boiling Point: 68.107 °C at 760 mmHg; (20)Vapour Pressure: 153.455 mmHg at 25 °C.
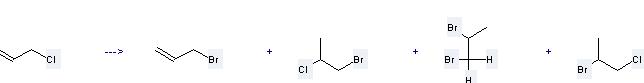
Preparation of 3-Bromopropene: this chemical can be prepared by 3-Chloro-propene. This reaction needs reagent HBr and solvent CH2Cl2 at temperature of 25 °C. The reaction time is 0.5 hours. The yield is 2 %.
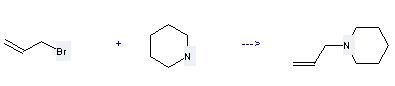
Uses of 3-Bromopropene: it is used to produce other chemicals. For example, it can react with Piperidine to get 1-Allyl-piperidine. The reaction occurs with solvent benzene at temperature of 95-100 °C. The yield is 37 %.
When you are dealing with this chemical, you should be very careful. This chemical may destroy living tissue on contacting. In addition, This chemical is highly flammable and it may catch fire in contact with air and only need briefly contact with an ignition source. So it may cause burns. What's more, it is toxic by inhalation and if swallowed. Therefore, you should wear suitable protective clothing, gloves and eye/face protection. And in case of contacting with eyes, you should rinse immediately with plenty of water and seek medical advice. This material and its container must be disposed of as hazardous waste.
You can still convert the following datas into molecular structure:
(1) SMILES: C=CCBr
(2) InChI: InChI=1S/C3H5Br/c1-2-3-4/h2H,1,3H2
(3) InChIKey: BHELZAPQIKSEDF-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LC50 | inhalation | 4110mg/m3 (4110mg/m3) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 18(4), Pg. 55, 1974. | |
| mouse | LD50 | intraperitoneal | 48mg/kg (48mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 20(12), Pg. 52, 1976. | |
| rat | LC50 | inhalation | 10gm/m3/30M (10000mg/m3) | Fiziologicheski Aktivnye Veshchestva. Physiologically Active Substances. Vol. 7, Pg. 35, 1975. | |
| rat | LD50 | oral | 120mg/kg (120mg/kg) | JAT, Journal of Applied Toxicology. Vol. 9, Pg. 235, 1989. |
Related Products
- ALLYL α-IONONE
- Allyl (2-methylbutoxy)acetate
- Allyl (3-methylbutoxy)acetate
- Allyl 2-(acetylamino)-2-deoxy-3-O-benzyl--D-glucopyranoside
- Allyl 2-acetamido-2-deoxy-beta-D-glucopyranoside
- Allyl 2-chloroethylsulfide
- Allyl 2-ethylbutyrate
- Allyl 2-oxocyclopentanecarboxylate
- Allyl 3,4-epoxy-6-methylcyclohexanecarboxylate
- Allyl 3,5,5-trimethylhexanoate
- 106956-32-5
- 1069-57-4
- 106966-25-0
- 1069-66-5
- 106966-55-6
- 106-96-7
- 106-97-8
- 1069-79-0
- 106981-52-6
- 106981-59-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View