-
Name
Isophthalic acid
- EINECS 204-506-4
- CAS No. 121-91-5
- Article Data269
- CAS DataBase
- Density 1.451 g/cm3
- Solubility water: 0.01 g/100 mL (25 °C)
- Melting Point 341-343 °C(lit.)
- Formula C8H6O4
- Boiling Point 378.274 °C at 760 mmHg
- Molecular Weight 166.133
- Flash Point 196.749 °C
- Transport Information
- Appearance white to light yellow crytal power
- Safety 24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Isophthalicacid (8CI);3-Carboxybenzoic acid;NSC 15310;m-Benzenedicarboxylic acid;m-Carboxybenzoic acid;m-Dicarboxybenzene;m-Phthalic acid;1,3-Benzenedicarboxylicacid;
- PSA 74.60000
- LogP 1.08300
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dimethanol benzene With (6-di-tert-butylphosphinomethyl-2,2’-bipyridyl)Ru(CO)HCl; water; sodium hydroxide for 18h; Inert atmosphere; Reflux; Stage #2: Acidic conditions; | 100% |
| With C24H33IrN4O3; water; sodium hydroxide for 18h; Reflux; | 97% |
| With sodium tetrahydroborate; 1% Pd/C; water; potassium hydroxide In methanol at 20℃; for 9h; In air; | 89% |
| Multi-step reaction with 2 steps 1: concentrated hydrobromic acid 2: aqueous potassium permanganate View Scheme |
-
-
16034-14-3
isophthalic Acid dibenzyl ester

-
-
108-88-3
toluene

-
A
-
121-91-5
isophthalic acid

-
B
-
620-47-3
1-methyl-3-(phenylmethyl)-benzene

-
C
-
620-83-7
1-methyl-4-(phenylmethyl)benzene

-
D
-
713-36-0
2-benzyltoluene

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; water at 80℃; for 2h; regioselective reaction; | A 99% B n/a C n/a D n/a |

-
-
201230-82-2
carbon monoxide

-
-
585-76-2
m-bromobenzoic acid

-
A
-
121-91-5
isophthalic acid

-
B
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; cobalt(II) oxide at 65℃; under 1520 Torr; for 1h; Irradiation; | A 98% B 1.5% |
| With sodium hydroxide; cobalt(II) oxide at 65℃; under 1520 Torr; for 1h; Product distribution; Irradiation; other cobalt salt catalysts and various aromatic halides investigated; | A 98% B 1.5% |
| With sodium methylate; cobalt(II) acetate In methanol at 65℃; under 1520 Torr; for 1h; Irradiation; | A 72.6% B 21.3% |


| Conditions | Yield |
|---|---|
| With phosphoric acid; ozone In water; acetonitrile at 25℃; for 29h; pH=4 - 4.5; Temperature; UV-irradiation; | 96% |
| With oxygen; 1-hydroxy-pyrrolidine-2,5-dione; cobalt(II) acetate In acetic acid at 60℃; under 12049.9 Torr; for 1h; Product distribution / selectivity; | 80% |
| With oxygen In chloroform for 12h; Irradiation; | 75% |
-
-
108-38-3
m-xylene

-
A
-
121-91-5
isophthalic acid

-
B
-
619-21-6
m-formylphenyl benzoic acid

-
C
-
99-04-7
m-Toluic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In dimethyl sulfoxide at 70℃; for 10h; Catalytic behavior; Reagent/catalyst; Overall yield = > 99 %; | A 96% B n/a C n/a |
| With cobalt(II) acetate; manganese(II) acetate; acetic acid; 3-benzyl-1-methylimidazolium bromide at 215℃; for 3h; |


| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 115℃; for 5h; | 96% |

| Conditions | Yield |
|---|---|
| With potassium ferrate(VI) In neat (no solvent) for 5h; Milling; | A n/a B 92.8% |
-
-
201230-82-2
carbon monoxide

-
-
626-00-6
1,3-Diiodobenzene

-
A
-
121-91-5
isophthalic acid

-
B
-
618-51-9
3-Iodobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; amphiphilic resin-supported phosphine-palladium; water at 25℃; under 760 Torr; for 12h; hydroxycarbonylation; | A 92% B n/a |


| Conditions | Yield |
|---|---|
| With sodium perborate In acetic acid at 45 - 50℃; | 90% |
| With potassium hydroxide; sodium tetrahydroborate; air; palladium on activated charcoal In methanol at 20℃; for 6h; | 88% |
| With potassium permanganate |

| Conditions | Yield |
|---|---|
| With oxygen; manganese (II) acetate tetrahydrate; cobalt(II) diacetate tetrahydrate; 1N,3N,5N-trihydroxy-1,3,5-triazin-2,4,6[1H,3H,5H]-trione In acetic acid at 120℃; under 760.051 Torr; for 15h; | 89% |
-
-
95-63-6
1,2,4-Trimethylbenzene

-
A
-
121-91-5
isophthalic acid

-
B
-
100-21-0
terephthalic acid

-
C
-
528-44-9
1,2,4-benzene tricarboxylic acid

| Conditions | Yield |
|---|---|
| With oxygen; titanium(IV) isopropylate; tetrabutoxytitanium; manganese(II) acetate; cobalt(II) acetate; ammonium bromide; cerous nitrate In water; acetic acid at 150 - 225℃; under 5250.53 - 18751.9 Torr; for 1.2 - 1.25h; Product distribution / selectivity; | A n/a B n/a C 88.3% |

-
-
108-36-1
1,3-dibromobenzene

-
-
18414-58-9
diphenylmethylsilanecarboxylic acid

-
A
-
121-91-5
isophthalic acid

-
B
-
585-76-2
m-bromobenzoic acid

| Conditions | Yield |
|---|---|
| With lithium trimethylsilanolate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene; bis(dibenzylideneacetone)-palladium(0) In 1,4-dioxane at 80℃; for 16h; Glovebox; Overall yield = 40 mg; | A 88% B 7% |

-
-
626-18-6
1,3-dimethanol benzene

-
A
-
121-91-5
isophthalic acid

-
B
-
28286-79-5
3-(Hydroxymethyl)benzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dimethanol benzene With cesiumhydroxide monohydrate; C24H19F3N7O3RuS(1+)*CF3O3S(1-) In water at 150℃; for 24h; Inert atmosphere; Schlenk technique; Stage #2: With hydrogenchloride In water Inert atmosphere; Schlenk technique; | A 88% B 10% |
| Stage #1: 1,3-dimethanol benzene With cesiumhydroxide monohydrate; C24H19F3N7O3RuS(1+)*CF3O3S(1-) In water at 150℃; for 24h; Inert atmosphere; Schlenk technique; Stage #2: With hydrogenchloride In water Inert atmosphere; Schlenk technique; | A 23% B 52% |

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; air; cobalt(II) acetate; manganese(II) acetate In acetic acid at 150℃; under 22801.5 Torr; for 3h; | A 87% B 7% |
| Stage #1: m-xylene With hafnium(IV) oxide; N-hydroxy-o-sulphonyl benzamide; C32H12F4FeN8; C44H28N4O8Ru; oxygen at 145℃; under 6000.6 Torr; for 1.6h; Stage #2: With hafnium(IV) oxide; N-hydroxy-o-sulphonyl benzamide; C32H12F4FeN8; C44H28N4O8Ru; oxygen; acetic acid at 176℃; under 13501.4 Torr; for 2.2h; Temperature; Pressure; Reagent/catalyst; | A 84.4% B 15.6% |
| With N-hydroxyphthalimide; oxygen; nitric acid at 110℃; under 760.051 Torr; for 6h; Ionic liquid; | A 71% B n/a |
-
-
19806-17-8
1,3-benzenediacetic acid

-
-
121-91-5
isophthalic acid

| Conditions | Yield |
|---|---|
| With iodine; dimethyl sulfoxide at 120℃; for 26h; Sealed tube; Green chemistry; | 87% |
-
-
95-47-6
o-xylene

-
-
106-42-3
para-xylene

-
-
108-38-3
m-xylene

-
A
-
121-91-5
isophthalic acid

-
B
-
100-21-0
terephthalic acid

-
C
-
88-99-3
benzene-1,2-dicarboxylic acid

-
D
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; oxygen; manganese(II) bromide In water at 400℃; under 187519 Torr; Product distribution / selectivity; | A 86.3% B 66.7% C 61.5% D n/a |

-
-
67-56-1
methanol

-
-
201230-82-2
carbon monoxide

-
-
108-36-1
1,3-dibromobenzene

-
A
-
121-91-5
isophthalic acid

-
B
-
1459-93-4
dimethyl Isophthalate

-
C
-
618-89-3
methyl 3-bromobenzoate

| Conditions | Yield |
|---|---|
| With tert-Amyl alcohol; sodium hydride; cobalt(II) acetate In tetrahydrofuran at 40℃; under 760 Torr; for 14h; Irradiation; | A 5% B 86% C 8% |

-
-
201230-82-2
carbon monoxide

-
-
108-36-1
1,3-dibromobenzene

-
A
-
121-91-5
isophthalic acid

-
B
-
1459-93-4
dimethyl Isophthalate

-
C
-
618-89-3
methyl 3-bromobenzoate

| Conditions | Yield |
|---|---|
| With methanol; tert-Amyl alcohol; sodium hydride; cobalt(II) acetate In tetrahydrofuran at 40℃; under 760 Torr; for 14h; Irradiation; | A 5% B 86% C 8% |


-
-
121-91-5
isophthalic acid

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 150℃; | 85.4% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; cobalt(II) acetate at 80℃; under 1520 Torr; for 2.5h; Irradiation; | 81.6% |
| With sodium hydroxide; dicobalt octacarbonyl In water at 65℃; under 1471.02 Torr; for 6h; Product distribution; Irradiation; | 94.0 % Chromat. |
-
-
180044-88-6
C26H16N2O4

-
-
121-91-5
isophthalic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 2h; | 80% |

| Conditions | Yield |
|---|---|
| With anthracene; oxygen; acetic acid; hydrogen bromide; cobalt(II) acetate; manganese(II) acetate In water at 180 - 195℃; under 21446.5 Torr; for 1h; Product distribution / selectivity; | A n/a B 80% C 0.8% |
| With oxygen; acetic acid; hydrogen bromide; cobalt(II) acetate; manganese(II) acetate In water at 180 - 195℃; under 21446.5 Torr; for 1h; Product distribution / selectivity; | A n/a B 73% C 1% |

-
-
108-67-8
1,3,5-trimethyl-benzene

-
A
-
121-91-5
isophthalic acid

-
B
-
554-95-0
benzene-1,3,5-tricarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water; dihydrogen peroxide; manganese(II) bromide at 380℃; under 172517 Torr; | A n/a B 78% |
-
-
108-70-3
1,3,5-trichlorobenzene

-
-
201230-82-2
carbon monoxide

-
A
-
121-91-5
isophthalic acid

-
B
-
554-95-0
benzene-1,3,5-tricarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide; cobalt(II) acetate In ethanol at 65℃; under 1520 Torr; for 18h; Irradiation; | A 21.3% B 76.4% |

-
-
201230-82-2
carbon monoxide

-
-
150177-91-6
3-bromo-1-iodylbenzene

-
A
-
591-18-4
1-Bromo-3-iodobenzene

-
B
-
121-91-5
isophthalic acid

-
C
-
585-76-2
m-bromobenzoic acid

| Conditions | Yield |
|---|---|
| With sodium tetrachloropalladate; sodium carbonate In water at 40℃; for 5.5h; Yields of byproduct given; | A n/a B 14% C 76% |

-
-
201230-82-2
carbon monoxide

-
-
150177-91-6
3-bromo-1-iodylbenzene

-
A
-
625-99-0
3-iodochlorobenzene

-
B
-
121-91-5
isophthalic acid

-
C
-
585-76-2
m-bromobenzoic acid

| Conditions | Yield |
|---|---|
| With sodium tetrachloropalladate; sodium carbonate In water at 40℃; for 5.5h; Yields of byproduct given; | A n/a B 14% C 76% |


| Conditions | Yield |
|---|---|
| Stage #1: malononitrile; 3-Iodobenzoic acid With copper(l) iodide; caesium carbonate; L-proline In dimethyl sulfoxide at 130℃; for 24h; Ullmann type reaction; Inert atmosphere; Stage #2: In dimethyl sulfoxide at 140℃; for 12h; Stage #3: With hydrogenchloride In water pH=2 - 3; | 70% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; water; dihydrogen peroxide; manganese(II) bromide at 380℃; under 172517 Torr; | A 66% B n/a |
| With dihydrogen peroxide; oxygen; manganese(II) bromide In water at 400℃; under 187519 Torr; Product distribution / selectivity; | A 58% B n/a |

| Conditions | Yield |
|---|---|
| Stage #1: carbon dioxide With o-phenylenebis(diphenylphosphine); copper(II) acetate monohydrate In 1,4-dioxane at 65℃; for 0.416667h; Schlenk technique; Stage #2: 1-Bromo-3-iodobenzene With palladium diacetate; triethylamine; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane; toluene at 100℃; Schlenk technique; | 65% |

| Conditions | Yield |
|---|---|
| With boron trifluoride at 65℃; for 0.333333h; | 100% |
| With thionyl chloride at 20℃; for 72h; | 95.7% |
| With 4-methyl-morpholine; 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride for 3h; | 92% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 80℃; for 4h; | 100% |
| With thionyl chloride In N,N-dimethyl acetamide; acetonitrile at 120℃; for 1h; Solvent; Large scale; | 99.4% |
| With thionyl chloride In N,N-dimethyl-formamide | 98% |
-
-
121-91-5
isophthalic acid


| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; bromo-tris(1-pyrrolidinyl)phosphonium hexafluorophosphate In N,N-dimethyl-formamide at 25℃; for 16h; Condensation; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine; triphenyl phosphite; lithium chloride In 1-methyl-pyrrolidin-2-one at 100℃; for 3h; | 100% |
-
-
75-44-5
phosgene

-
-
121-91-5
isophthalic acid

-
A
-
1459-93-4
dimethyl Isophthalate

-
B
-
99-63-8
benzene-1,3-dicarbonyl dichloride

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane | A n/a B 100% |

-
-
121-91-5
isophthalic acid

-
-
2052-49-5
tetra(n-butyl)ammonium hydroxide

-
-
68124-65-2, 149182-00-3
bis(tetrabutylammonium) isophthatale

| Conditions | Yield |
|---|---|
| In methanol; water at 20℃; | 100% |
| In methanol at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate at 170 - 220℃; for 4.5h; Large scale; | 99% |
| In 5,5-dimethyl-1,3-cyclohexadiene at 160℃; for 2h; | 51% |

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate at 170 - 220℃; for 4.5h; Large scale; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-diaza-bicyclo[2.2.2]octane; zinc(II) nitrate hexahydrate; isophthalic acid In N,N-dimethyl-formamide for 1h; Stage #2: 1,2-pentanediol In N,N-dimethyl-formamide at 130℃; for 48h; | 99% |


| Conditions | Yield |
|---|---|
| Stage #1: 1,4-diaza-bicyclo[2.2.2]octane; zinc(II) nitrate hexahydrate; isophthalic acid In N,N-dimethyl-formamide for 1h; Stage #2: propylene glycol In N,N-dimethyl-formamide at 130℃; for 48h; | 99% |


| Conditions | Yield |
|---|---|
| at 230℃; for 6h; | 98% |
| With polyphosphoric acid at 200℃; for 6h; Inert atmosphere; | 94% |
| With phosphoric acid at 250℃; for 4h; | 84% |
-
-
121-91-5
isophthalic acid

-
-
122350-19-0
3,3'-carbonylbis<5-phenyl-1,3,4-oxadiazole-2(3H)-thione>

-
A
-
3004-42-0
5-phenyl-1,3,4-oxadiazole-2(3H)-thione

| Conditions | Yield |
|---|---|
| With pyridine In various solvent(s) for 4h; Ambient temperature; | A n/a B 98% C n/a |

-
-
553-26-4
4,4'-bipyridine

-
-
121-91-5
isophthalic acid

-
-
859149-40-9, 894073-63-3
[Zn(isophthalate)(4,4'-bipyridyl)]

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 119.84℃; for 24h; Inert atmosphere; | 98% |

-
-
884539-90-6
{(1R,2R)-2-[(-)-2-(3,5-di-tert-butyl-2-hydroxybenzylideneamino)cyclohexylimino]methyl}-6-tert-butylbenzene-1,4-diol

-
-
121-91-5
isophthalic acid

| Conditions | Yield |
|---|---|
| Stage #1: {(1R,2R)-2-[(-)-2-(3,5-di-tert-butyl-2-hydroxybenzylideneamino)cyclohexylimino]methyl}-6-tert-butylbenzene-1,4-diol; isophthalic acid With 4-(dimethylamino)pyridinium tosylate In dichloromethane at 20℃; for 0.25h; Schlenk technique; Inert atmosphere; Stage #2: With diisopropyl-carbodiimide at 20℃; for 40h; Schlenk technique; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With NaOH In water High Pressure; NdCl3*6H2O prepared by dissolution of Nd2O3 in hydrochloric acid mixed with H2bdc and phen , pH 5 (NaOH), sealed in a bomb, heated for 3 ds at 150°C, cooled to room temp.; ppt. filtered off, washed (ethanol), elem. anal.; | 96.4% |


| Conditions | Yield |
|---|---|
| With hydrogen; acetic acid; 5% rhodium on alumina In methanol at 20℃; under 2585.81 Torr; for 24h; | 96.3% |
| With hydrogen; acetic acid; Rh/Al2O3 In methanol at 20℃; under 2585.81 Torr; | 96.3% |
| With hydrogen; acetic acid In methanol | 96.3% |

| Conditions | Yield |
|---|---|
| tetrachlorobis(tetrahydrofuran)hafnium(IV) In o-xylene for 96h; Heating; | 96% |
-
-
121-91-5
isophthalic acid

-
-
101-80-4
4,4'-oxydiphenylene diamine

-
-
782504-25-0
bis(5-methyl-1,3-phenylene)-20-crown-6

| Conditions | Yield |
|---|---|
| With pyridine; triphenyl phosphite; lithium chloride In 1-methyl-pyrrolidin-2-one at 100℃; for 3h; | 96% |


| Conditions | Yield |
|---|---|
| Stage #1: 1,4-diaza-bicyclo[2.2.2]octane; zinc(II) nitrate hexahydrate; isophthalic acid In N,N-dimethyl-formamide for 1h; Stage #2: 1,2-dihydroxybutane In N,N-dimethyl-formamide at 130℃; for 48h; | 96% |


| Conditions | Yield |
|---|---|
| With borane-THF; boron trifluoride diethyl etherate In tetrahydrofuran at 20℃; for 18h; | 95% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 60℃; for 19h; | 94% |
| With lithium aluminium tetrahydride In tetrahydrofuran Heating; | 75% |
| With sodium tetrahydroborate In tetrahydrofuran; ethanol at 20℃; for 3h; | 69% |
| With sulfuric acid bei der elektrolytischen Reduktion; |

| Conditions | Yield |
|---|---|
| With pyridine; triphenyl phosphite In 1-methyl-pyrrolidin-2-one at 100℃; for 3h; | 95% |
-
-
121-91-5
isophthalic acid


| Conditions | Yield |
|---|---|
| With benzotriazol-1-yloxyl-tris-(pyrrolidino)-phosphonium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 23℃; for 18h; | 95% |
-
-
492-98-8
2,2'-biimidazole

-
-
121-91-5
isophthalic acid

-
-
7732-18-5
water

-
-
6147-53-1
cobalt(II) diacetate tetrahydrate

| Conditions | Yield |
|---|---|
| In water 2,2'-biimidazole added to a soln. of metal acetate in hot water, a soln.of isophthalic acid in hot water added; concn., controlled evapn. at room temp. for 2-3 wk; elem. anal.; | 94% |

Isophthalic acid Consensus Reports
Isophthalic acid Specification
The Isophthalic acid is an organic compound with the formula C8H6O4. The IUPAC name of this chemical is benzene-1,3-dicarboxylic acid. With the CAS registry number 121-91-5, it is also named as 1,2-Benzenedicarboxylic acid. The product's classification code is Skin / Eye Irritant. Mixed with terephthalic acid, isophthalic acid is used in the production of resins for drink bottles.
Physical properties about Isophthalic acid are: (1)ACD/LogP: 0.88 ; (2)ACD/BCF (pH 5.5): 1; (3)ACD/BCF (pH 7.4): 1; (4)ACD/KOC (pH 5.5): 1; (5)ACD/KOC (pH 7.4): 1; (6)#H bond acceptors: 4; (7)#H bond donors: 2; (8)#Freely Rotating Bonds: 2; (9)Polar Surface Area: 74.6 Å2; (10)Index of Refraction: 1.618; (11)Molar Refractivity: 40.113 cm3; (12)Molar Volume: 114.493 cm3; (13)Polarizability: 15.902×10-24cm3; (14)Surface Tension: 70.304 dyne/cm; (15)Density: 1.451 g/cm3; (16)Flash Point: 196.749 °C; (17)Enthalpy of Vaporization: 66.051 kJ/mol; (18)Boiling Point: 378.274 °C at 760 mmHg.
Preparation: this chemical can be prepared by benzene-1,3-dicarbaldehyde. This reaction will need reagent potassium permanganate.
.gif)
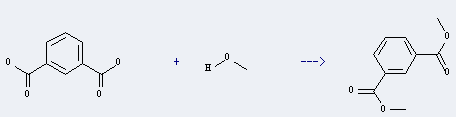
Uses of Isophthalic acid: it can be used to produce isophthalic acid dimethyl ester. It will need reagent hydrogen chloride.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: c1ccc(c(c1)C(=O)O)C(=O)O
(2)InChI: InChI=1/C8H6O4/c9-7(10)5-3-1-2-4-6(5)8(11)12/h1-4H,(H,9,10)(H,11,12)
(3)InChIKey: XNGIFLGASWRNHJ-UHFFFAOYAX
(4)Std. InChI: InChI=1S/C8H6O4/c9-7(10)5-3-1-2-4-6(5)8(11)12/h1-4H,(H,9,10)(H,11,12)
(5)Std. InChIKey: XNGIFLGASWRNHJ-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 4200mg/kg (4200mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: EXCITEMENT | Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. Vol. 246, Pg. 851, 1958. |
| rat | LD50 | oral | 10400mg/kg (10400mg/kg) | "Prehled Prumyslove Toxikologie; Organicke Latky," Marhold, J., Prague, Czechoslovakia, Avicenum, 1986Vol. -, Pg. 317, 1986. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View